Various Dry Shampoos
Allegations: Failing to disclose products contain the carcinogen benzene
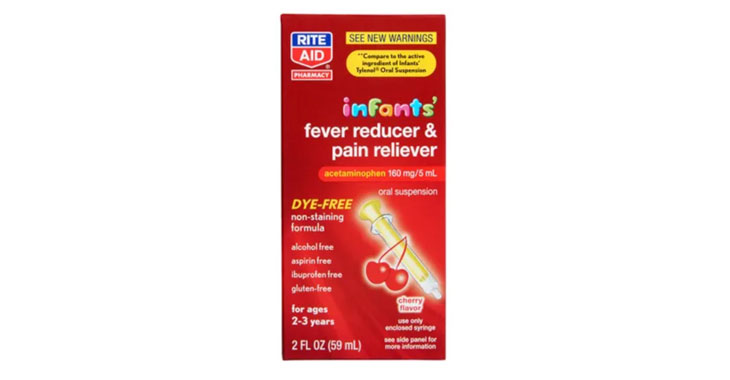
In July 2020, a class-action lawsuit was filed against Rite Aid for allegedly misleadingly advertising its Infants’ Fever Reducer & Pain Reliever and Children’s Fever Reducer & Pain Reliever as different products and charging more for the Infants’ medication when, according to plaintiffs, the medications contain the same amount of the active ingredient acetaminophen. (Ostermeier-McLucas et al v. Rite Aid Corp., Case No. 20-cv-2915, E.D.N.Y.)
For more of TINA.org’s coverage of the marketing of acetaminophen products, click here.
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Misrepresenting that it protects consumers’ personal information when it failed to do so and there was a data breach in June 2024
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing medicines as “Non-Drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing that products “promote a healthy mouth” when using them contributes to various oral health issues
Allegations: Misleadingly marketing Rite Aid pain relief patches
Allegations: Marketing products as safe pain relievers for pregnant women without warning consumers that scientific evidence shows prenatal exposure to APAP can cause neurodevelopmental disorders in children
Allegations: Misleadingly marketing products as “Maximum Strength” when there are other products that contain more lidocaine
Allegations: Failing to disclose that products may contain a dangerous substance that increases the risk of serious adverse health consequences and death
Allegations: Failing to disclose that products contain a harmful substance and may increase the risk of contracting invasive infections
An FDA panel’s recent findings has led to a flood of lawsuits.
Lawsuits claim infant-specific products aren’t any different than acetaminophen medications for older children.
Drugstore chain’s No. 1 doctor-recommended claims come down in wake of NAD inquiry.
These claims are tough to swallow.
Unproven cold prevention and treatment claims are nothing to sneeze at.