Various Dry Shampoos
Allegations: Failing to disclose products contain the carcinogen benzene
September 2019: A federal judge dismissed certain claims but allowed the false advertising claims to move forward.
November 2018: A class-action lawsuit was filed against Rite Aid for allegedly misleadingly marketing its Acetaminophen Rapid Release Gelcaps as being comparable to Tylenol® Extra Strength Rapid Release Gels when, according to plaintiffs, the Tylenol® Gels have “unique laser drilled holes” that the Rite Aid Gelcaps do not have. In addition, the complaint alleges that the Rite Aid gels are “rapid release” when, according to plaintiffs, the medicine does not work faster than non-rapid release products. (Bailey et al v. Rite Aid Corp., Case No. 18-cv-6926, N.D. Cal.)
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Misrepresenting that it protects consumers’ personal information when it failed to do so and there was a data breach in June 2024
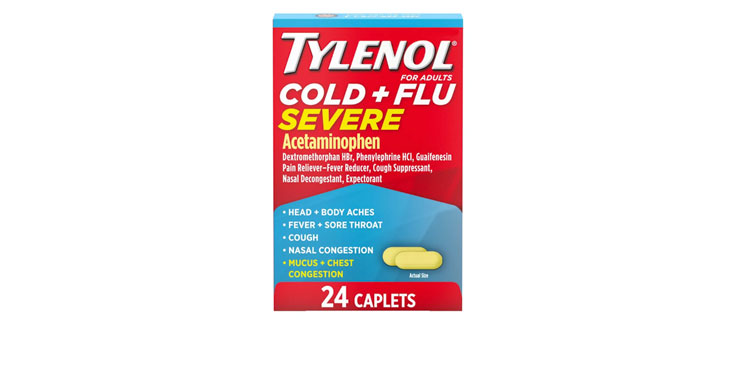
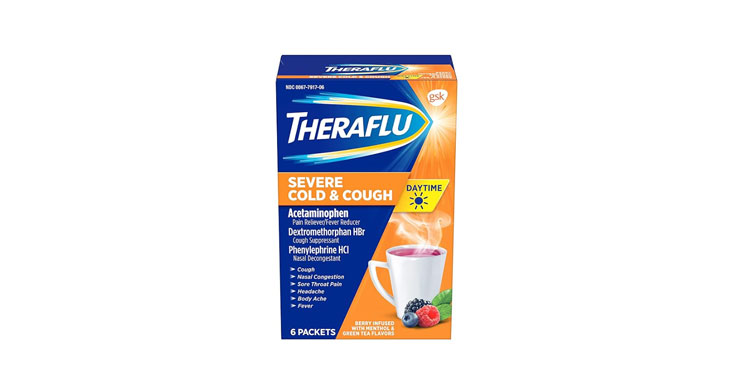
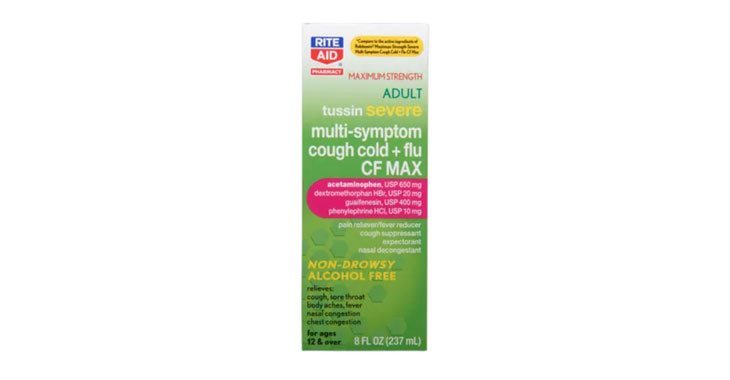
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing medicines as “Non-Drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing that products “promote a healthy mouth” when using them contributes to various oral health issues
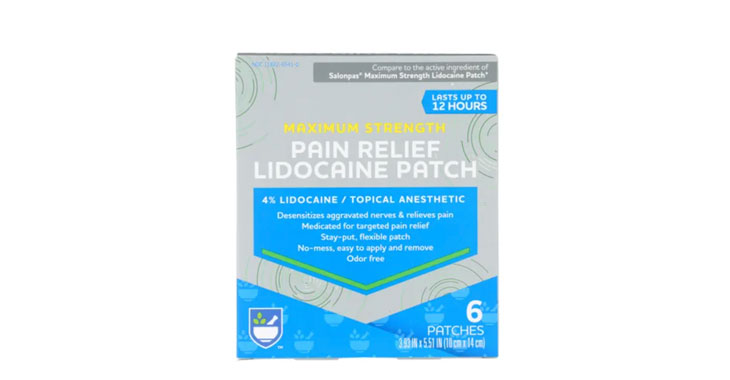
Allegations: Misleadingly marketing Rite Aid pain relief patches
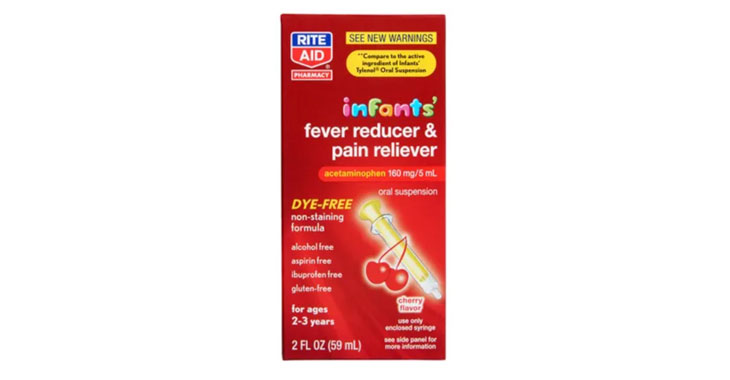
Allegations: Marketing products as safe pain relievers for pregnant women without warning consumers that scientific evidence shows prenatal exposure to APAP can cause neurodevelopmental disorders in children
Allegations: Misleadingly marketing products as “Maximum Strength” when there are other products that contain more lidocaine
Allegations: Failing to disclose that products may contain a dangerous substance that increases the risk of serious adverse health consequences and death
Allegations: Failing to disclose that products contain a harmful substance and may increase the risk of contracting invasive infections
An FDA panel’s recent findings has led to a flood of lawsuits.
Lawsuits claim infant-specific products aren’t any different than acetaminophen medications for older children.
Drugstore chain’s No. 1 doctor-recommended claims come down in wake of NAD inquiry.
These claims are tough to swallow.
Unproven cold prevention and treatment claims are nothing to sneeze at.