Various Dry Shampoos
Allegations: Failing to disclose products contain the carcinogen benzene
Neu et al. v. Rite Aid Corp.
23-cv-3242, E.D.N.Y.
(April 2023)
Rite Aid Maximum Strength Pain Relief Lidocaine Patch
Falsely advertising that patches last up to 12 hours when they cannot adhere to skin for more than 4 hours and often peel off within minutes of light activity
Misleadingly marketing products as maximum strength when other products deliver more lidocaine
Falsely marketing that patches “Desensitize Aggravated Nerves” when patches are not capable of providing such relief
Pending
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Misrepresenting that it protects consumers’ personal information when it failed to do so and there was a data breach in June 2024
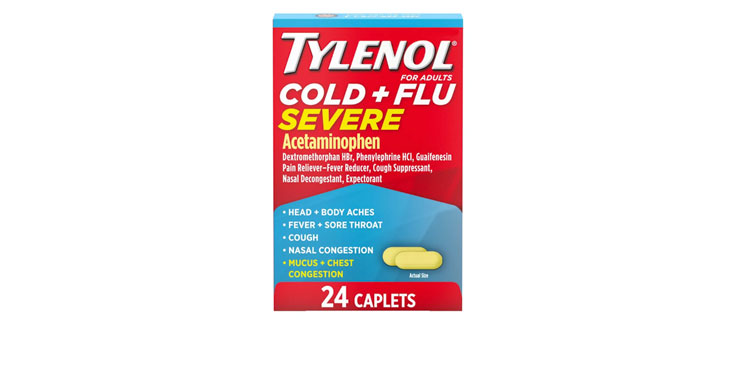
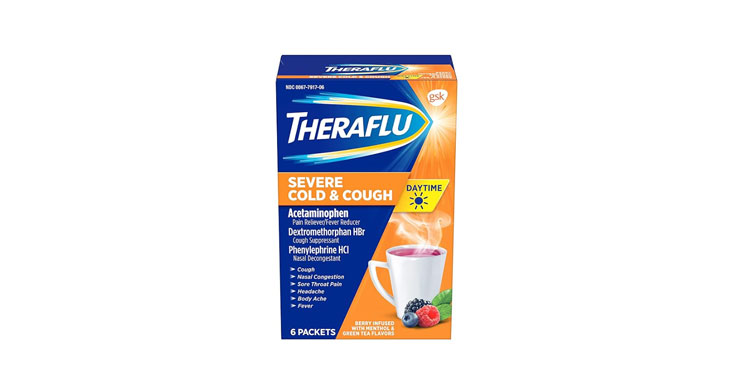
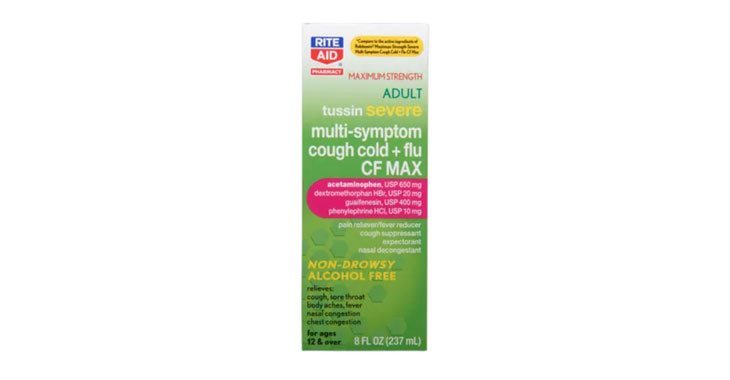
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing medicines as “Non-Drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing that products “promote a healthy mouth” when using them contributes to various oral health issues
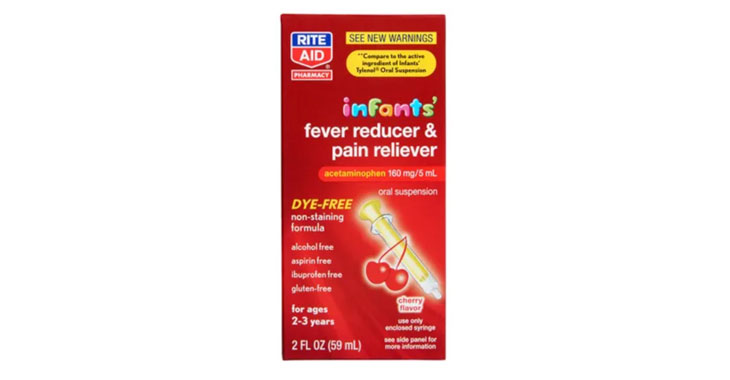
Allegations: Marketing products as safe pain relievers for pregnant women without warning consumers that scientific evidence shows prenatal exposure to APAP can cause neurodevelopmental disorders in children
Allegations: Misleadingly marketing products as “Maximum Strength” when there are other products that contain more lidocaine
Allegations: Failing to disclose that products may contain a dangerous substance that increases the risk of serious adverse health consequences and death
Allegations: Failing to disclose that products contain a harmful substance and may increase the risk of contracting invasive infections
An FDA panel’s recent findings has led to a flood of lawsuits.
Lawsuits claim infant-specific products aren’t any different than acetaminophen medications for older children.
Drugstore chain’s No. 1 doctor-recommended claims come down in wake of NAD inquiry.
These claims are tough to swallow.
Unproven cold prevention and treatment claims are nothing to sneeze at.