Nutrafol Needs to Shed Its Deceptive Hair Growth Claims
Illegal claims that company's products prevent hair loss also need to go.
|

UPDATE 5/8/23: A class-action lawsuit that cites TINA.org’s findings has been filed against Nutrafol alleging the company falsely advertises its products as “clinically proven” to improve hair growth and prevent shedding, and makes unapproved health claims without properly disclosing that the products have not been evaluated by the FDA as required by federal law. Our original article follows.
If Nutrafol were looking for permission from its new parent company to continue making deceptive hair growth claims – first flagged by TINA.org in early 2020 – it might’ve found it in a May 2022 press release from its new owner, consumer goods giant Unilever.
In the release announcing its acquisition of the hair health brand, Unilever touted Nutrafol’s “hair growth products” as “clinically backed” and “clinically tested,” which conveys the same message to consumers as “clinically proven.” Which is to say, it signals there is competent scientific evidence that proves the products are effective – which is just the kind of evidence that Nutrafol does not possess.
So perhaps it’s not surprising that, under Unilever’s new ownership, Nutrafol in the last six months has amplified its deceptive marketing message that its supplements and serum for both men and women are “clinically proven” to increase hair growth and prevent hair loss.
Not only that, numerous Nutrafol influencers are failing to properly disclose their connection to the company or label their promotional posts as paid ads, as required by federal law, with some influencers also using a new awareness campaign created by Nutrafol called Shed the Silence to supplement their pitch.
But it starts with the unauthorized claims at the center of the company’s multimillion-dollar deceptive marketing campaign.
Prohibited drug claims
Take away the “clinically proven” claims and Nutrafol would still be violating the law.
The FDA has established that claims to increase hair growth and prevent hair loss are drug – or disease-treatment – claims requiring its approval, which Nutrafol acknowledges it does not have.
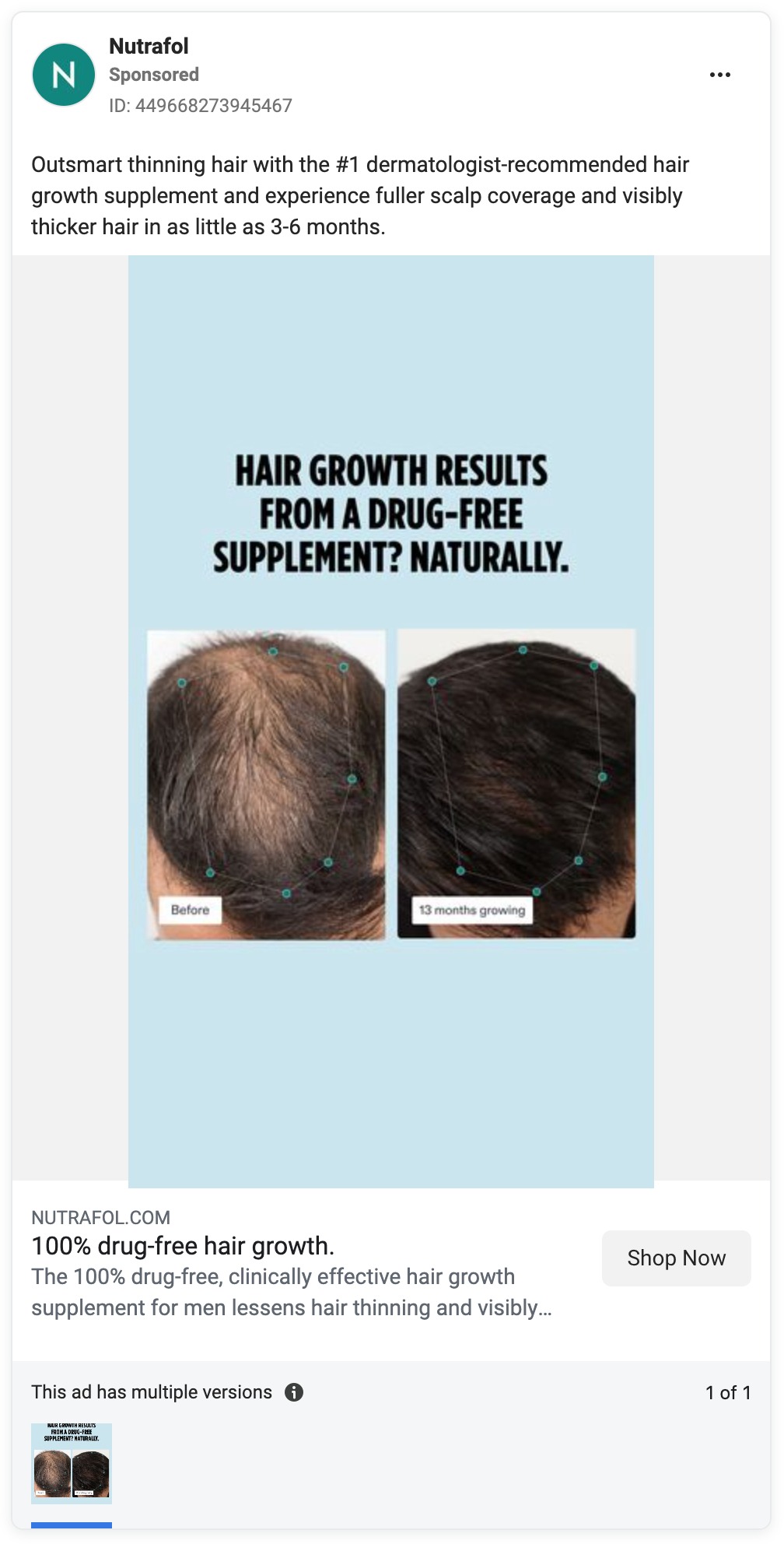
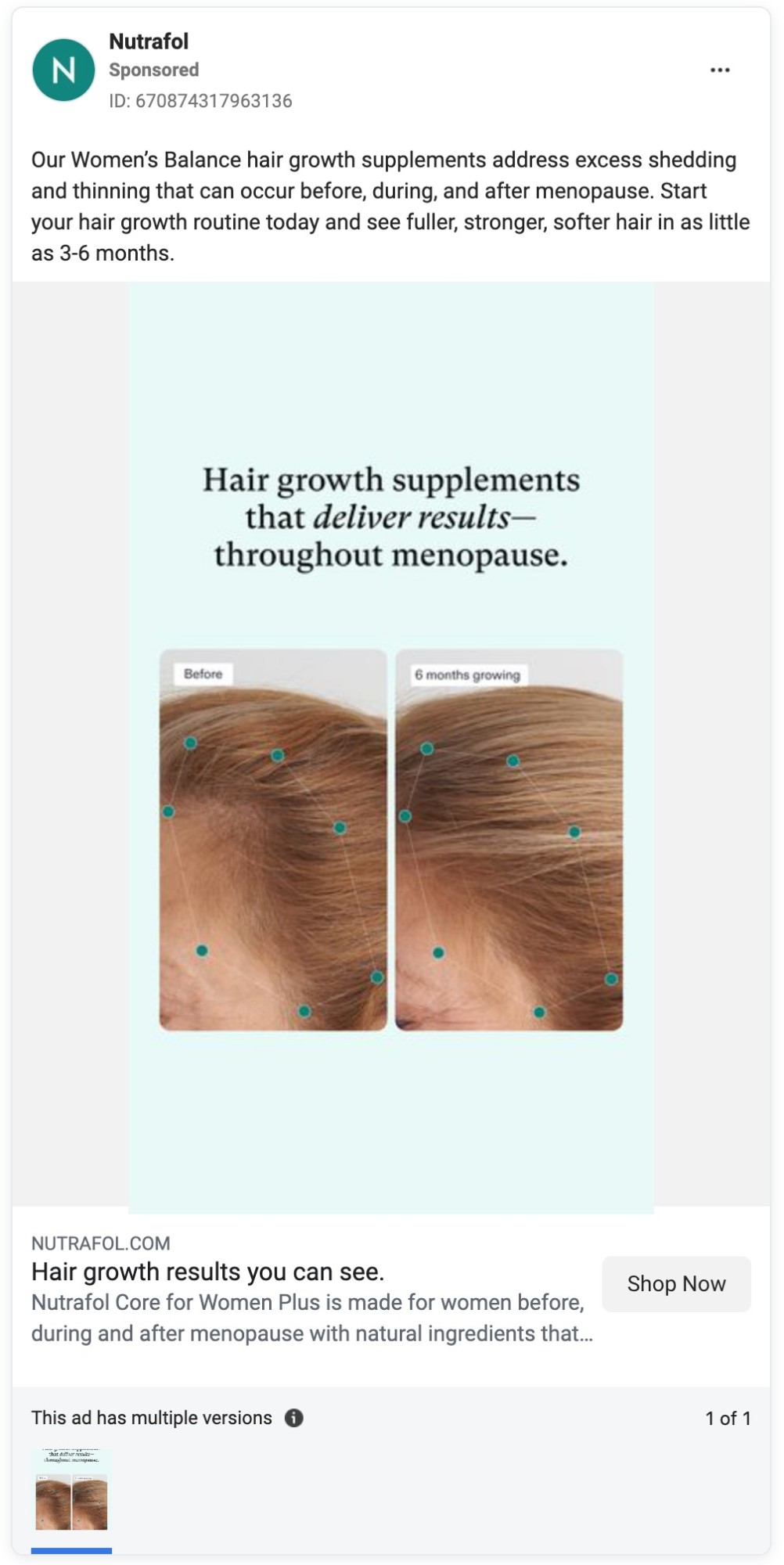
Yet Nutrafol’s marketing, from its website to its TV commercials to its social media and influencer ads, is awash in hair growth claims. And hair loss prevention claims are not far behind.
For example, since the Unilever acquisition, Nutrafol has continued to pour millions of dollars into a national TV ad titled “Hair Growth Journey,” according to data provided by iSpot.tv, which tracks ad spending.
The ad features people who experienced hair thinning and who said that after they started taking Nutrafol, within months they noticed their hair becoming stronger and thicker. The ad refers to Nutrafol as a “clinically effective … hair growth supplement” and ends with the punchline: “Start your hair growth journey at Nutrafol.com.”
The company has also run dozens of ads on Facebook in recent months promoting its products for hair growth.
Here are some examples of the company’s unapproved health claims.
#Ad disclosure issues
In addition, TINA.org has compiled a sampling of more than 30 influencer ads for Nutrafol in which the influencer fails to clearly and conspicuously disclose their material connection to the company, in violation of FTC law.
In many of the posts identified by TINA.org, the advertising disclosure is buried at the end of a long caption, while other posts flagged by TINA.org use Instagram’s built-in disclosure tool, which the FTC has said is not necessarily sufficient on its own at communicating a relationship with a brand.
What’s in Nutrafol?
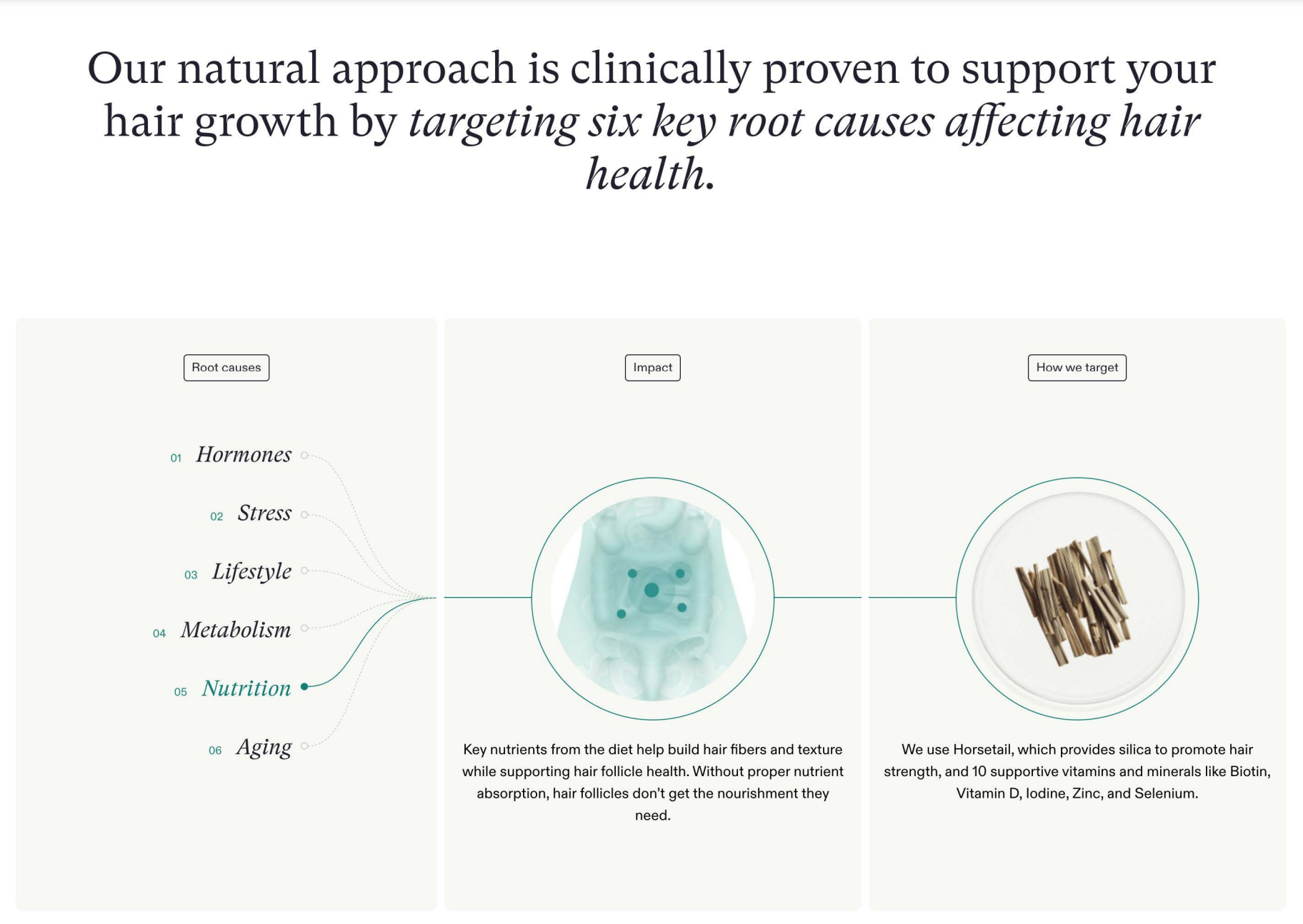
For starters, not finasteride (aka Propecia) or minoxidil (aka Rogaine) – the only two products that have been approved by the FDA to increase hair growth and treat different types of hair loss. (In fact, Nutrafol is marketed as an alternative to Propecia and Rogaine, as well as surgery.) Rather, Nutrafol’s supplements and “growth activator” hair serum contain a mix of plant-based “medical-grade ingredients” including ashwagandha, curcumin and pea sprouts.
The company’s supplements cost $88 for a one month’s supply and its serum is priced at $69 for a one to three months’ supply, according to Nutrafol.
Clinically proven? Not so much
While the FDA requires its approval to make disease-treatment claims, the FTC demands marketers have competent and reliable scientific evidence to substantiate such claims, which Nutrafol is also missing.

Some of the flaws in the four studies that Nutrafol lists on its website – and that the company and its influencers often cite as support for their illegal hair growth and hair loss prevention claims – include:
- Biased researchers who are employees of the company or promoters of its products.
- The exclusion of subjects with hair loss disorders (even as Nutrafol’s entire marketing campaign is centered around treating hair loss).
- The exclusion of subjects who reported high levels of stress or who recently experienced a stressful incident (yet Nutrafol markets products for hair loss attributable to stress, among five other “key root causes” including hormones and aging).
- The exclusion of subjects who were nursing or pregnant (meanwhile, Nutrafol and its influencers promote the company’s products for postpartum use).
- A placebo group that actually experienced a decrease in hair shedding.
- A reliance on subjects’ self-perceived hair thinning.
- Small sample sizes.
In short, Nutrafol is playing fast and loose with what it claims to be clinical proof in order to promote a marketing message that simply isn’t accurate.
Controlling the conversation
Nutrafol bills Shed the Silence as an awareness campaign aimed at normalizing female hair struggles but make no mistake, it’s also a marketing campaign.
While Nutrafol states in a disclaimer at the bottom of its website that the “forum is not intended to treat, diagnose, or otherwise support medical issues such as … hair loss,” in posts inviting their followers to join the private Shed the Silence Facebook group, some Nutrafol influencers also tout the company’s products as a hair loss treatment.
And when asked if the campaign is meant to target a specific demographic of women, Nutrafol’s vice president of brand marketing told MediaPost in September:
It is a pretty broad range. Hair issues can happen to a wide set of women for different reasons. It can be from stress to changes in hormones, diet and nutrition. …
An interesting statement from a company whose products are marketed to help with hair issues caused by stress, hormones and nutrition.
Meanwhile, on the Shed the Silence page, Nutrafol invites women “experiencing the emotional and social impact of hair changes” to “join the conversation” by becoming a member of the “Shed the Silence by Nutrafol” Facebook group.
But TINA.org joined the private group and found members who are Nutrafol influencers who do not make their material connection to the company known, including one woman who has posted multiple times in the group about Nutrafol products without disclosing she’s a Nutrafol partner, in violation of both federal law and alleged group rules that state “[u]sers may not promote the sale of any products.”
So while Nutrafol is encouraging women to join the conversation, it is also controlling it, while some of its influencers are using the group as a springboard to promote Nutrafol products.
The bottom line
A sophisticated company like Unilever, which knows what constitutes a drug claim, in addition to what is required for advertising substantiation, cannot claim ignorance over Nutrafol’s violations of the law. It’s time Unilever be the responsible parent and put an end to the deceptive marketing.
TINA.org reached out to Nutrafol and Unilever for comment. Check back for updates.
Find more of our coverage on Nutrafol here.
You Might Be Interested In

Most Deceptive Ads of 2023
Some of the worst ads TINA.org covered this year.

Hair-Raising Claims by Unilever’s Nutrafol Reported to Regulators
TINA.org files complaint with the FDA and FTC over company’s hair growth claims.

Consumer Alert: Hair Growth Products
Supplement and serum companies are targeting consumers experiencing hair loss.