GNC Total Lean Bars
Allegations: Misleadingly marketing products as “lean”
December 2014: After the complaint was amended in May 2014, the named plaintiff voluntarily dismissed the lawsuit with prejudice, meaning that the complaint cannot be refiled. The reasons for the dismissal have not yet been disclosed.
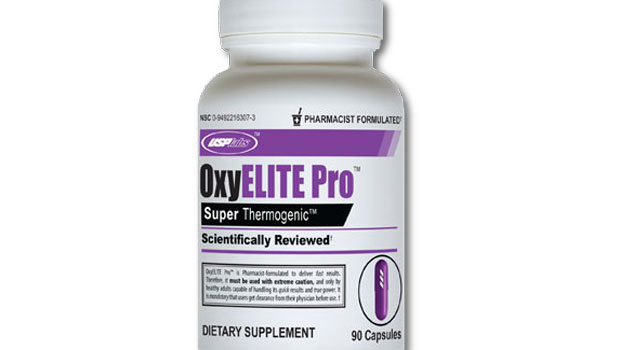
January 2014: A class-action lawsuit was filed against USPLabs and GNC for allegedly misleadingly labeling OxyElite Pro™ products, a variety energy and weight loss dietary supplement line containing aegeline. The complaint alleges, among other things, that the companies do not adequately warn consumers of the severe adverse health effects associated with the supplement. (Barot et al. v. USPLabs, LLC. and General Nutrition Center Holdings Inc., Case No. 14-cv-00562, D. NJ.).
For more information about other class action lawsuits filed against USPLabs and TINA.org’s coverage of the company, click here.
For more information about other class action lawsuits filed against GNC and TINA.org’s coverage of the company, click here.
Allegations: Misleadingly marketing products as “lean”
Allegations: Falsely marketing fish oil supplements
Allegations: Products do not provide the advertised benefits
Regulators send united message about deceptively marketed supplements.
DOJ files an indictment against USPlabs and top execs.
State officials demand Walmart, Target, Walgreens and GNC stop selling the supplements.
Agency prohibits products with DMAA from entering market.