Flintstones Gummies Sours
Allegations: Falsely advertising products as free of artificial flavors
March 2019: A federal judge denied the company’s motion to dismiss. To read the full decision, click here.
November 2017: A false advertising class-action lawsuit was filed against Bayer Healthcare for allegedly falsely marketing Flintstones™ Gummies Complete Children’s Multivitamin Supplement and Flintstones™ Sour Gummies Complete Children’s Multivitamin Supplement as being “Complete” multivitamin supplements when, according to plaintiffs, the products are missing several vitamins, including Vitamin K, Vitamin B1 (thiamine), Vitamin B2 (riboflavin), and Vitamin B3 (niacin). (Cabrera et al v. Bayer Healthcare LLC and Bayer Corp., Case No. 17-cv-8525, C. D. CA.)
Allegations: Falsely advertising products as free of artificial flavors
Allegations: Misleadingly marketing products as if one gummy provides consumers’ with their requisite daily nutrients
Allegations: Failing to disclose that products contain dangerously high levels of the carcinogen benzene
Allegations: Misleadingly marketing products as if the number of gummies in one bottle equals the number of daily servings
False advertising class-action lawsuits filed regarding the marketing of Seresto flea and tick collars
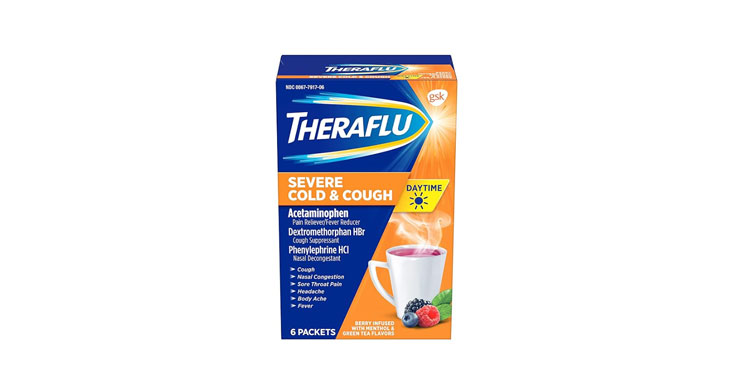
Allegations: Misleadingly claiming that products contain honey and lemon zest when the ingredients list reveals they don’t contain either Misleadingly marketing products as being for “severe cold & flu” when…
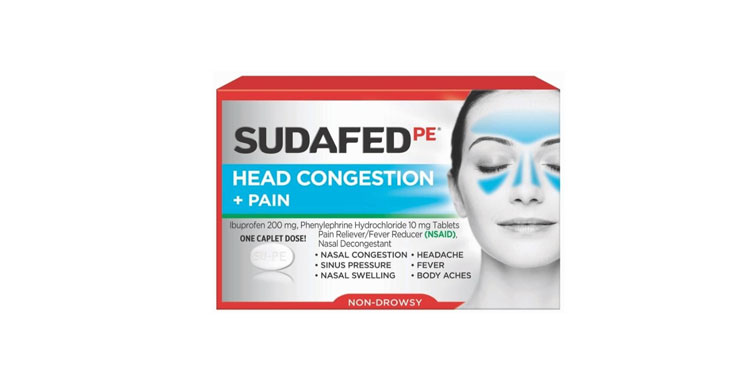
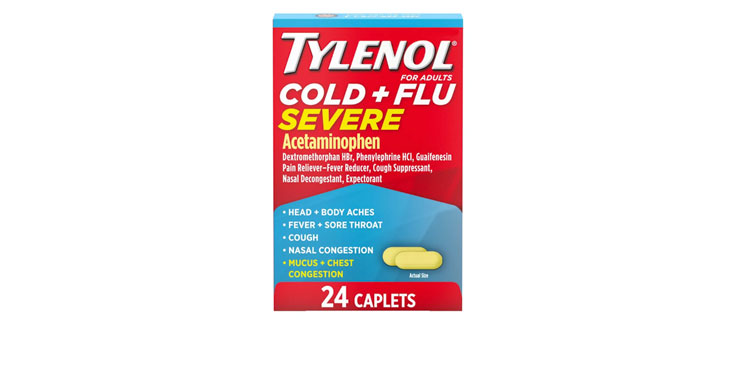
Allegations: Falsely marketing that phenylephrine products relieve nasal congestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing the products treat nasal congestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that products combat congestion and other sinus issues
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Failing to disclose sunscreens contain the carcinogen benzene
Allegations: False natural claims
Allegations: Misleadingly marketing the gummies as if one chewable provides the nutrients represented on the product label without adequately disclosing that the serving size is two gummies
Allegations: False natural claims
Allegations: Falsely marketing products as “non-drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing sunscreens as mineral-based when they often contain more chemical active ingredients than mineral active ingredients
Allegations: Marketing sunscreens as “safe and gentle on a baby’s skin” when they contain the carcinogen benzophenone
Allegations: Falsely representing that the active ingredient “targets an enzyme found in plants but not people or pets”
Allegations: Failing to warn consumers of the health risks associated with using the product due to its active ingredient
Allegations: Failing to warn consumers that ingredients may cause cancer
Allegations: Failing to warn consumers of the health risks associated with using the product due to its active ingredient (glyphosate)
Allegations: Falsely marketing that multivitamins provide various health benefits
Don’t confuse this product name for the serving size.
An FDA panel’s recent findings has led to a flood of lawsuits.
Hangover relief claims are disease-treatment claims requiring FDA approval.
Bayer finally agrees to drop “nothing works faster” claim following review.
Watchdog group files suit over Bayer’s claims about vitamins’ ability to improve health.