Flintstones Gummies Sours
Allegations: Falsely advertising products as free of artificial flavors
May 2015: The named plaintiffs voluntarily dismissed this action When a complaint is dismissed without prejudice, an amended version of the complaint can be refiled.. The reasons have not been disclosed.
October 2014: Another class-action lawsuit was filed against Bayer Healthcare for allegedly falsely advertising Flintstone Healthy Brain Support, a dietary supplement containing Omega-3 DHA, as supporting healthy brain function without scientific evidence to support such claim. (Kaplan et al v. Bayer Healthcare, LLC, Case No. 14-cv-23789, S. D. FL.).
For more information about other class-action lawsuits against Bayer Healthcare and TINA.org’s coverage of the company, click here.
Allegations: Falsely advertising products as free of artificial flavors
Allegations: Misleadingly marketing products as if one gummy provides consumers’ with their requisite daily nutrients
Allegations: Failing to disclose that products contain dangerously high levels of the carcinogen benzene
Allegations: Misleadingly marketing products as if the number of gummies in one bottle equals the number of daily servings
False advertising class-action lawsuits filed regarding the marketing of Seresto flea and tick collars
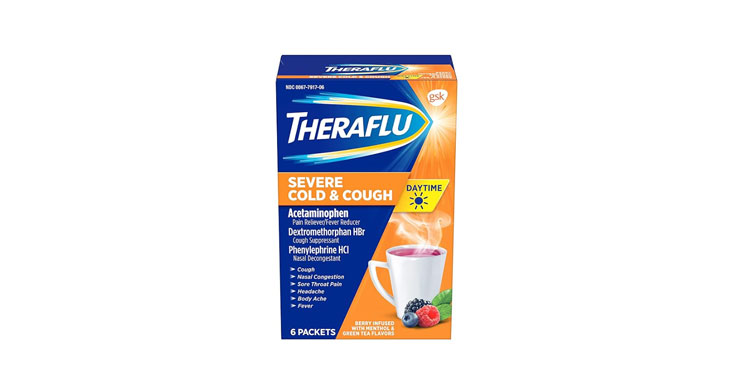
Allegations: Misleadingly claiming that products contain honey and lemon zest when the ingredients list reveals they don’t contain either Misleadingly marketing products as being for “severe cold & flu” when…
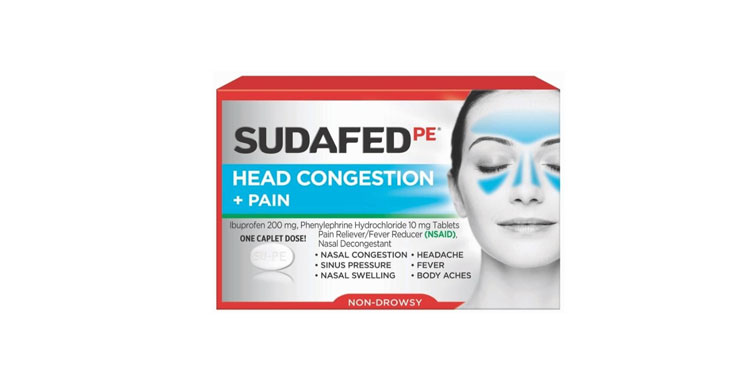
Allegations: Falsely marketing that phenylephrine products relieve nasal congestion
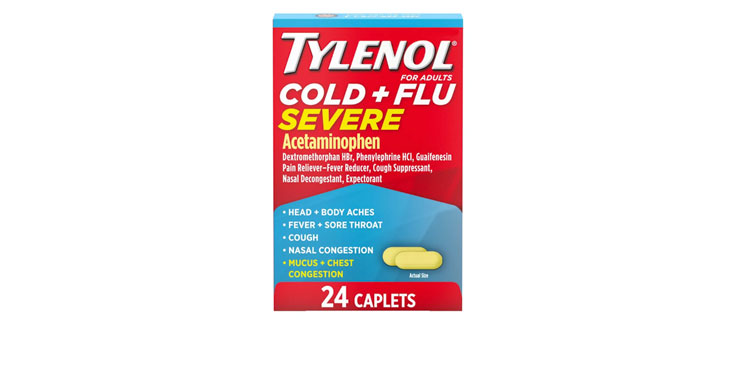
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing the products treat nasal congestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that products combat congestion and other sinus issues
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Failing to disclose sunscreens contain the carcinogen benzene
Allegations: False natural claims
Allegations: Misleadingly marketing the gummies as if one chewable provides the nutrients represented on the product label without adequately disclosing that the serving size is two gummies
Allegations: False natural claims
Allegations: Falsely marketing products as “non-drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing sunscreens as mineral-based when they often contain more chemical active ingredients than mineral active ingredients
Allegations: Marketing sunscreens as “safe and gentle on a baby’s skin” when they contain the carcinogen benzophenone
Allegations: Falsely representing that the active ingredient “targets an enzyme found in plants but not people or pets”
Allegations: Failing to warn consumers of the health risks associated with using the product due to its active ingredient
Allegations: Failing to warn consumers that ingredients may cause cancer
Allegations: Failing to warn consumers of the health risks associated with using the product due to its active ingredient (glyphosate)
Allegations: Falsely marketing that multivitamins provide various health benefits
DOJ alleges Bayer violated previous court order by making unsupported claims about product.
Are FTC settlements just a cost of doing business?