Flintstones Gummies Sours
Allegations: Falsely advertising products as free of artificial flavors
Parker et al. v. Bayer Corp., GSK Consumer Healthcare, Inc., Rite Aid Corp., and Walgreen Co.
23-cv-3663, E.D. Penn.
(Sept. 2023)
Alka-Seltzer Plus Severe Cold+Flu, Theraflu Severe Cold & Cough, Rite Aid Multi-Symptom Cold & Flu Relief, Rite Aid Sinus Pressure & Congestion Relief PE, and Walgreens Sinus Pressure & Pain
Falsely marketing that medicines treat nasal congestion when the active ingredient (phenylephrine) is not an effective decongestant
Pending
Allegations: Falsely advertising products as free of artificial flavors
Allegations: Misleadingly marketing products as if one gummy provides consumers’ with their requisite daily nutrients
Allegations: Failing to disclose that products contain dangerously high levels of the carcinogen benzene
Allegations: Misleadingly marketing products as if the number of gummies in one bottle equals the number of daily servings
False advertising class-action lawsuits filed regarding the marketing of Seresto flea and tick collars
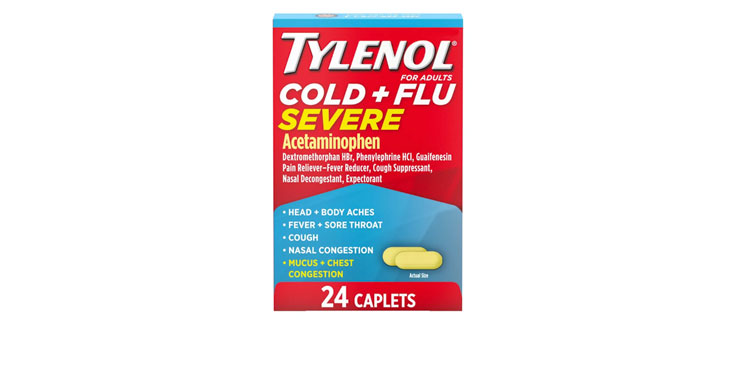
Allegations: Misleadingly claiming that products contain honey and lemon zest when the ingredients list reveals they don’t contain either Misleadingly marketing products as being for “severe cold & flu” when…
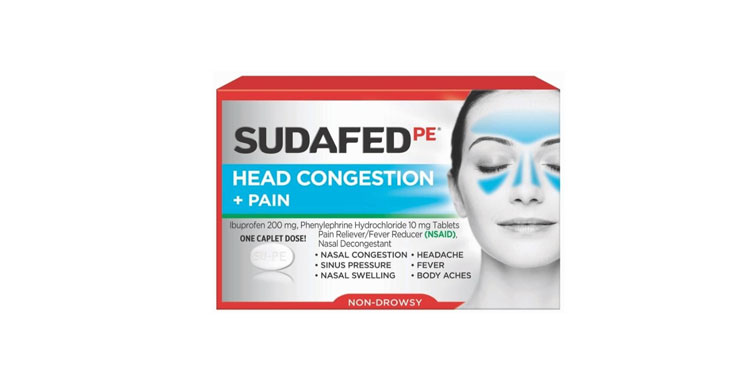
Allegations: Falsely marketing that phenylephrine products relieve nasal congestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing the products treat nasal congestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that products combat congestion and other sinus issues
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Failing to disclose sunscreens contain the carcinogen benzene
Allegations: False natural claims
Allegations: Misleadingly marketing the gummies as if one chewable provides the nutrients represented on the product label without adequately disclosing that the serving size is two gummies
Allegations: False natural claims
Allegations: Falsely marketing products as “non-drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing sunscreens as mineral-based when they often contain more chemical active ingredients than mineral active ingredients
Allegations: Marketing sunscreens as “safe and gentle on a baby’s skin” when they contain the carcinogen benzophenone
Allegations: Falsely representing that the active ingredient “targets an enzyme found in plants but not people or pets”
Allegations: Failing to warn consumers of the health risks associated with using the product due to its active ingredient
Allegations: Failing to warn consumers that ingredients may cause cancer
Allegations: Failing to warn consumers of the health risks associated with using the product due to its active ingredient (glyphosate)
Allegations: Falsely marketing that multivitamins provide various health benefits
Don’t confuse this product name for the serving size.
An FDA panel’s recent findings has led to a flood of lawsuits.
FDA targets companies selling eye drops illegally marketed to treat conditions like pink eye.
Hangover relief claims are disease-treatment claims requiring FDA approval.
Plaintiffs allege packaging misrepresents lidocaine dosages as ‘maximum strength,’ among other things.