February 2021: This case was transferred from a court in New York to one in Illinois. (Case No. 21-cv-856, N.D. Ill.)
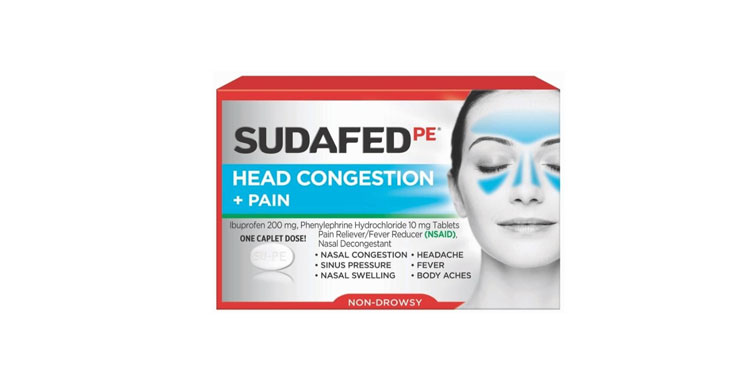
September 2020: A class-action lawsuit was filed against Walgreens for allegedly falsely marketing that its Fast-Release Quick Gels™ acetaminophen products are comparable to Tylenol® Extra Strength Rapid Release Gels when, according to plaintiffs, the Tylenol products have “unique laser drilled holes” that the Walgreens products do not have. The complaint also claims that the Walgreens gels are “fast-release” when, according to plaintiffs, the medicine does not work faster than other products that are not marketed as “fast-release.” (Gray et al v. Walgreens Boots Alliance, Inc. and LNK International, Inc., Case No. 20-cv-4415, E.D.N.Y.)
For more of TINA.org’s coverage of the marketing of medicine’s, click here.