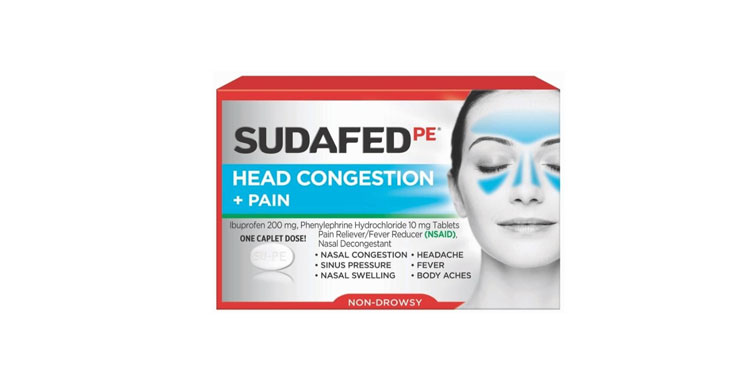
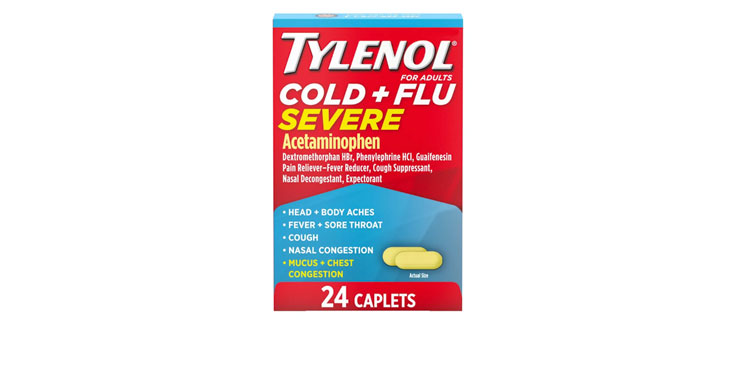
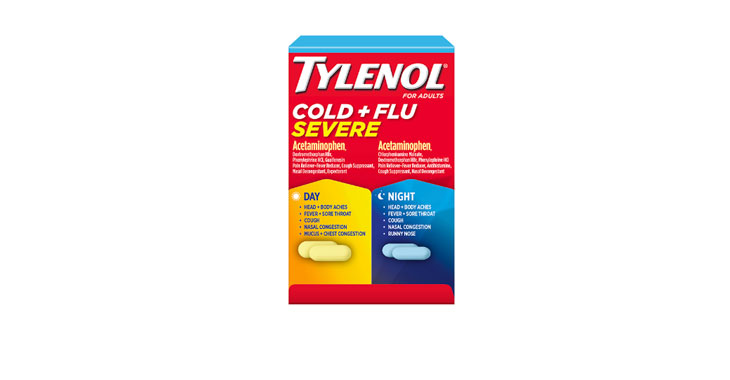
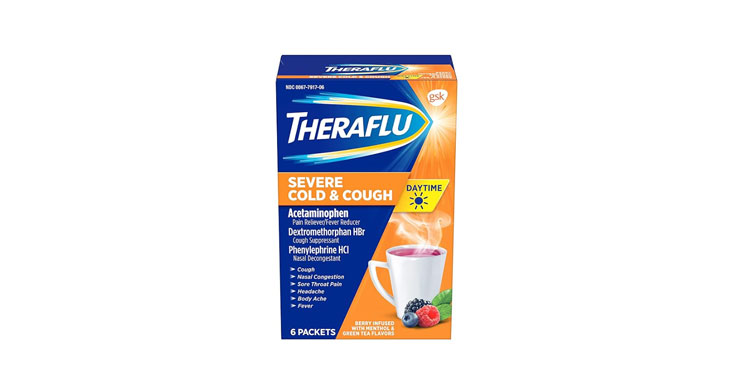
Walgreens Men’s Testosterone Complex
Allegations: Misleadingly marketing that products boost free testosterone levels, increase lean muscle mass, strength, and endurance, and improve sexual function in men when none of the ingredients provide the advertised…