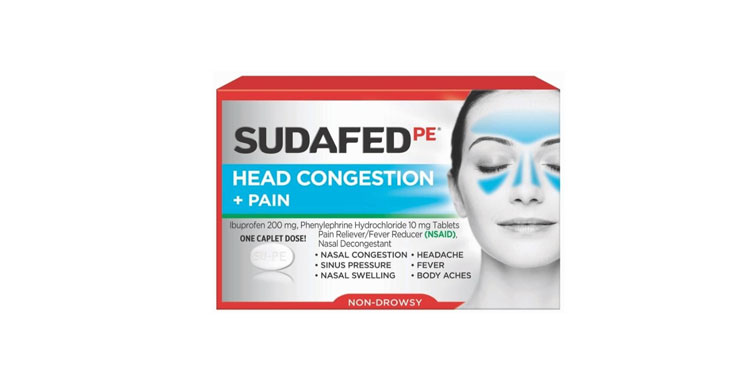
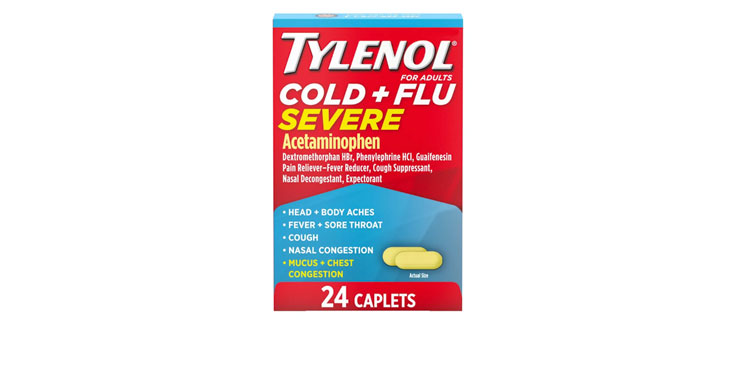
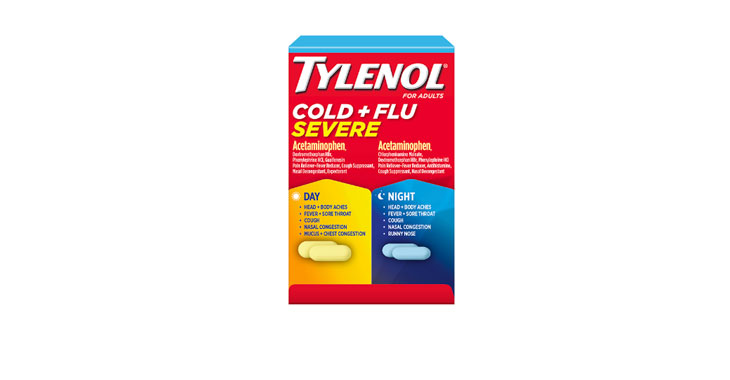
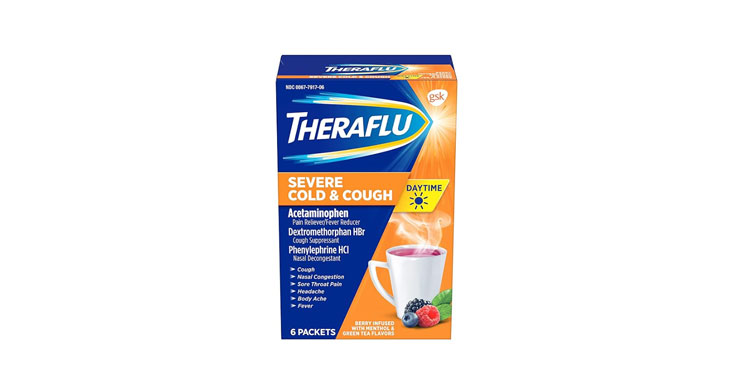
In February 2020, a class-action lawsuit was filed against Walgreens for allegedly misleadingly marketing Nice! Honey Graham Crackers. Specifically, the complaint claims that the retailer misleadingly represents that the main sweetener in the crackers is honey and the primary flour ingredient is whole grain graham flour when, according to the plaintiffs, the ingredients list shows that sugar is the primary sweetener and the crackers contain more “enriched flour” than “graham flour.” Plaintiffs also allege that the honey in the crackers is used as a coloring ingredient to make them look like they contain more whole grain graham flour than they actually do and the company gives consumers the impression that honey is a flavoring ingredient without adequately disclosing that the crackers also contain other flavoring ingredients. (Brown et al v. Walgreen Co., Case No. 20-cv-891, S.D.N.Y.)
For more of TINA.org’s coverage of the marketing of graham crackers, click here.