Neuriva
Allegations: Falsely marketing that products have been “clinically tested” to provide various brain health benefits
Grimsley et al. v. Reckitt Benckiser, LLC
23-cv-24588, N.D. Fla.
(Sept. 2023)
Mucinex Fast-Max Day Cold & Flu and Night Cold & Flu
Falsely marketing that medicines treat congestion when the active ingredient (phenylephrine) is not an effective decongestant
Pending
Allegations: Falsely marketing that products have been “clinically tested” to provide various brain health benefits
Allegations: Falsely marketing products as safe, healthy, nutritious, and committed to reducing their environmental impact
Allegations: Deceptively marketing products as “nutritionally appropriate” for children between nine and eighteen months
Allegations: Falsely marketing products as safe when they are dangerous for premature infants
Allegations: Failing to disclose that products contain PFAS
Allegations: Misleadingly marketing products as safe when they contained, or were at risk of containing, the carcinogen benzene
Allegations: Failing to disclose that products contain toxic heavy metals
Allegations: Misleadingly representing that products were safe and tested by dermatologists when they contained, or were at risk of containing, the carcinogen benzene
Allegations: Misleadingly marketing that the detergent “kills 99.9% of bacteria”
Allegations: Deceptively marketing that detergents bring color back to clothing
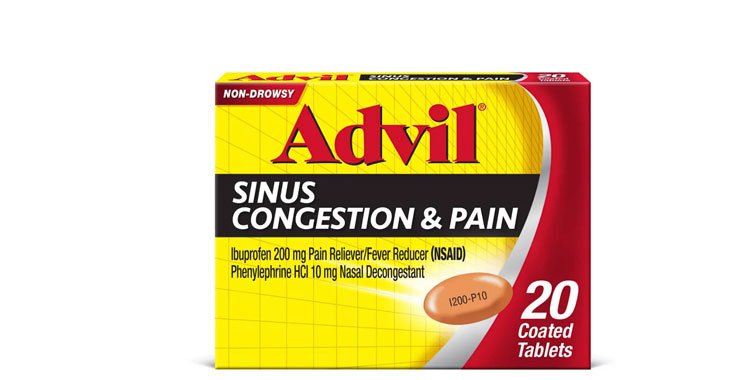
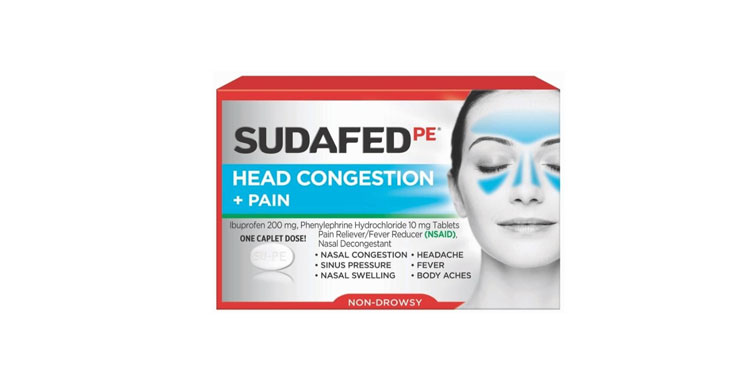
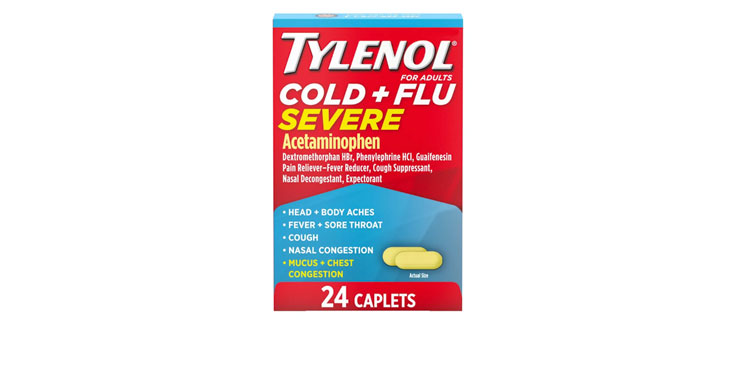
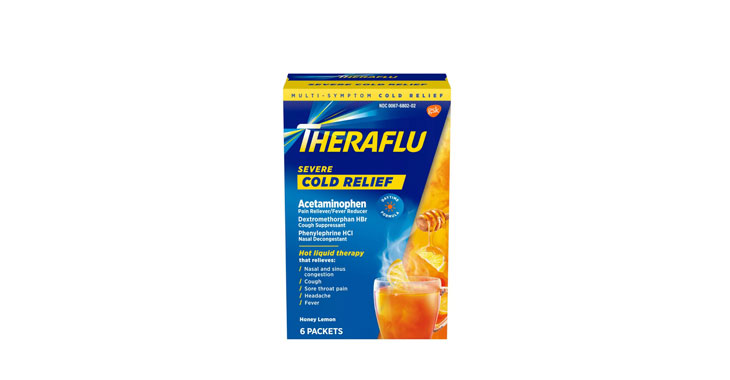
Allegations: Falsely marketing that phenylephrine products treat nasal congestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines are decongestants
Allegations: Falsely marketing that products provide relief from congestion, sinus pressure and other symptoms
Allegations: Falsely marketing the products treat nasal congestion
Allegations: Falsely marketing that products contain sandalwood essential oil
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing that products treat nasal congestion
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that medicines relieve nasal congestion and sinus pressure
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines treat congestion and other sinus issues
Allegations: Falsely marketing Neuriva supplements as “clinically proven” to improve “brain performance” when the company has no scientific or clinical proof that the supplements or ingredients in them provide the…
Allegations: Misleadingly marketing infant formulas as “Milk-based” when the primary ingredient is a form of sugar
Allegations: Products do not contain enough powder to make the advertised number of bottles
Allegations: Misleadingly marketing certain products as being for children and others as being for adults when the products contain the same amounts of the same ingredients
Allegations: Falsely advertising that Move Free provides joint health benefits when the ingredients in the supplement do not provide such benefits
Readers have told us to look into these MLMs.
TINA.org Joins with AARP in Objecting to Move Free Class-Action Settlement MADISON, Conn., March 12, 2015 — More than 46 million people suffer from arthritis, many of them elderly, and…
Consumers report adverse reactions.
Class-action settlement over supplement sold by Walmart, Walgreens and Supervalu provides little relief to consumers.