ChapStick Natural Claims
Allegations: False natural claims
Riccio et al. v. Pfizer, Inc.
23-cv-13843, N.D. Ill.
(Sept. 2023)
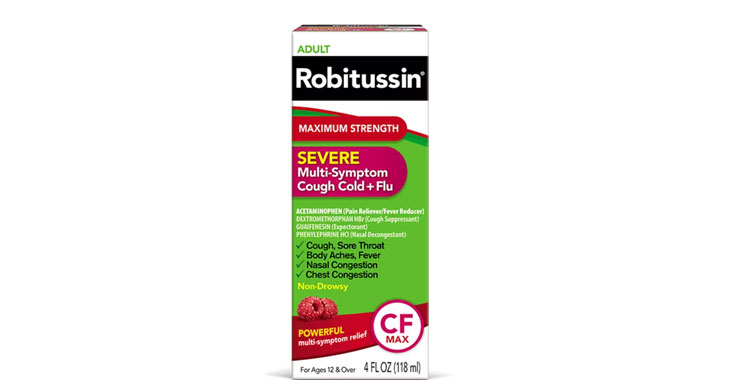
Robitussin Cold + Flu products
Falsely marketing that medicines relieve nasal congestion when the active ingredient (phenylephrine) is not an effective decongestant
Pending
Allegations: False natural claims
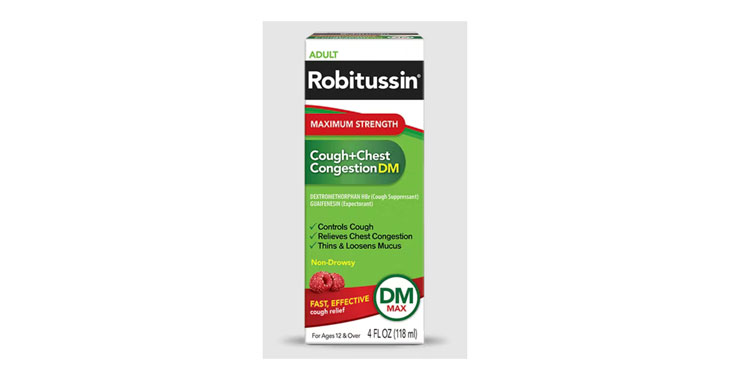
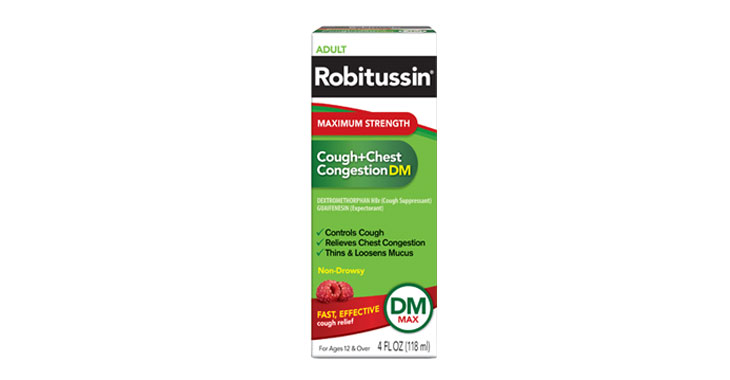
Allegations: Falsely marketing medicine as “Non-Drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing the ingredients in and the health benefits provided by products
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that medicines relieve nasal congestion and sinus pressure
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: False natural claims
Allegations: Falsely marketing products as non-drowsy when an ingredient in them causes drowsiness
Allegations: Deceptively marketing products as “Maximum Strength” when the dosage of the active ingredients is less than or the same as the regular strength products
An FDA panel’s recent findings has led to a flood of lawsuits.
Several products marketed as ‘non-drowsy’ contain an ingredient that causes drowsiness, lawsuits claim.
TINA.org’s election coverage focuses on the questionable ads running in between the news broadcasts.
Is Big Pharma marketing a drug to help aging men with low sex drives or really selling a made-up disease?
Justice Department says company put profits over safety.