ChapStick Natural Claims
Allegations: False natural claims
Woodhams et al v. Pfizer Inc.
18-cv-3990, S.D.N.Y.
(May 2018)
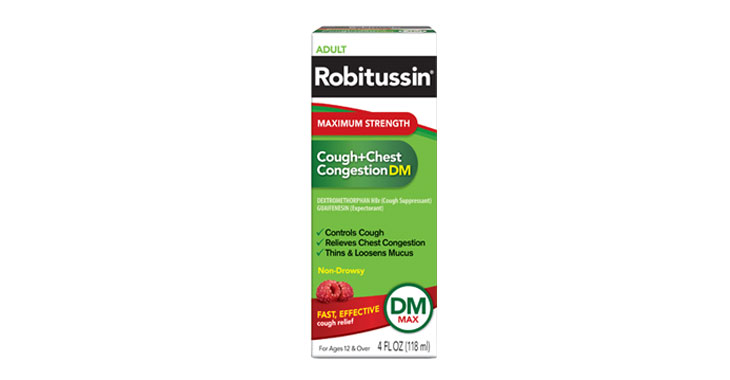
Maximum Strength Robitussin Cough+Chest Congestion DM
Deceptively marketing products as “Maximum Strength” when the dosage of the active ingredients is less than or the same as the regular strength products
Pending
Allegations: False natural claims
Allegations: Falsely marketing medicine as “Non-Drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing the ingredients in and the health benefits provided by products
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that medicines relieve nasal congestion and sinus pressure
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing that medicines relieve nasal congestion
Allegations: False natural claims
Allegations: Falsely marketing products as non-drowsy when an ingredient in them causes drowsiness
An FDA panel’s recent findings has led to a flood of lawsuits.
Several products marketed as ‘non-drowsy’ contain an ingredient that causes drowsiness, lawsuits claim.
TINA.org’s election coverage focuses on the questionable ads running in between the news broadcasts.
Is Big Pharma marketing a drug to help aging men with low sex drives or really selling a made-up disease?
Justice Department says company put profits over safety.