CATrends: OTC Cold and Flu Meds Falsely Marketed as Nasal Decongestants
An FDA panel’s recent findings has led to a flood of lawsuits.
An FDA panel’s recent findings has led to a flood of lawsuits.
Products marketed to clear up stuffy noses and relieve sinus congestion don’t work, researchers say.
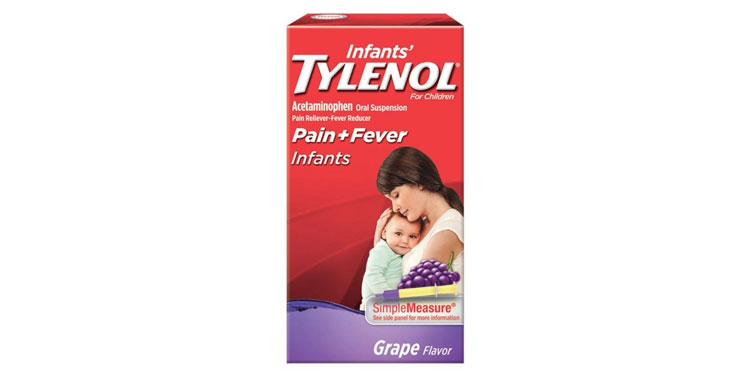
Lawsuits claim infant-specific products aren’t any different than acetaminophen medications for older children.
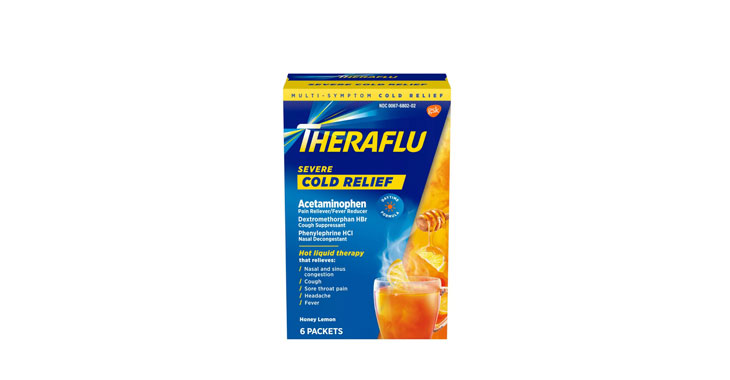
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that phenylephrine products treat nasal congestion
Allegations: Falsely marketing that medicines treat nasal congestion
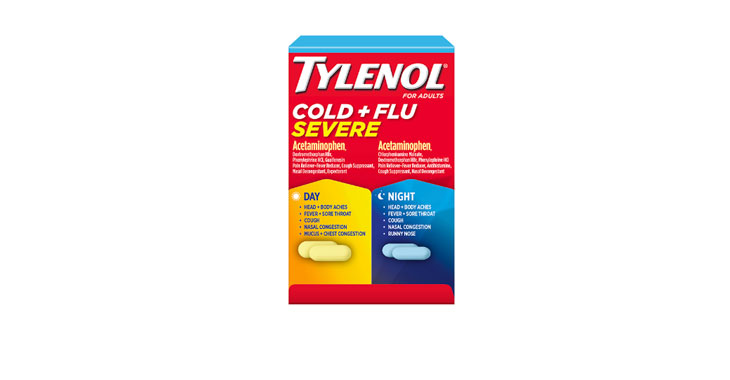
Allegations: Falsely marketing that medicines are decongestants
Allegations: Falsely marketing the products treat nasal congestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that medicines relieve nasal congestion
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing products as decongestants
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines treat nasal decongestion
Allegations: Falsely marketing that medicines treat congestion and other sinus issues
Allegations: Falsely advertising that products provide faster relief than other acetaminophen products
Allegations: Misleadingly marketing medicines as “non-drowsy” when an ingredient in them causes drowsiness
Allegations: Marketing products as safe without disclosing that they contain a harmful heavy metal
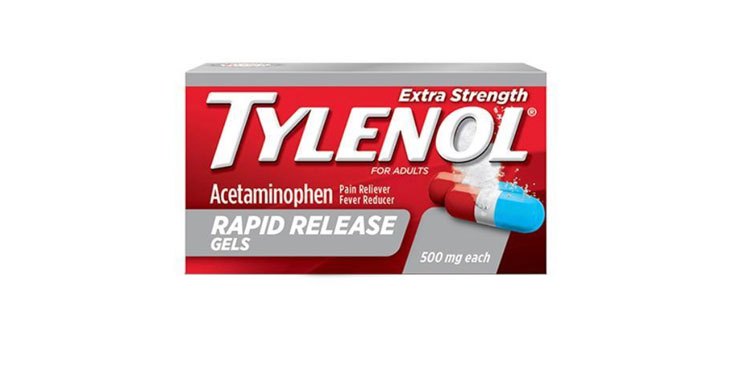
Allegations: Misleadingly marketing that Tylenol rapid release products work faster than other products when they don’t