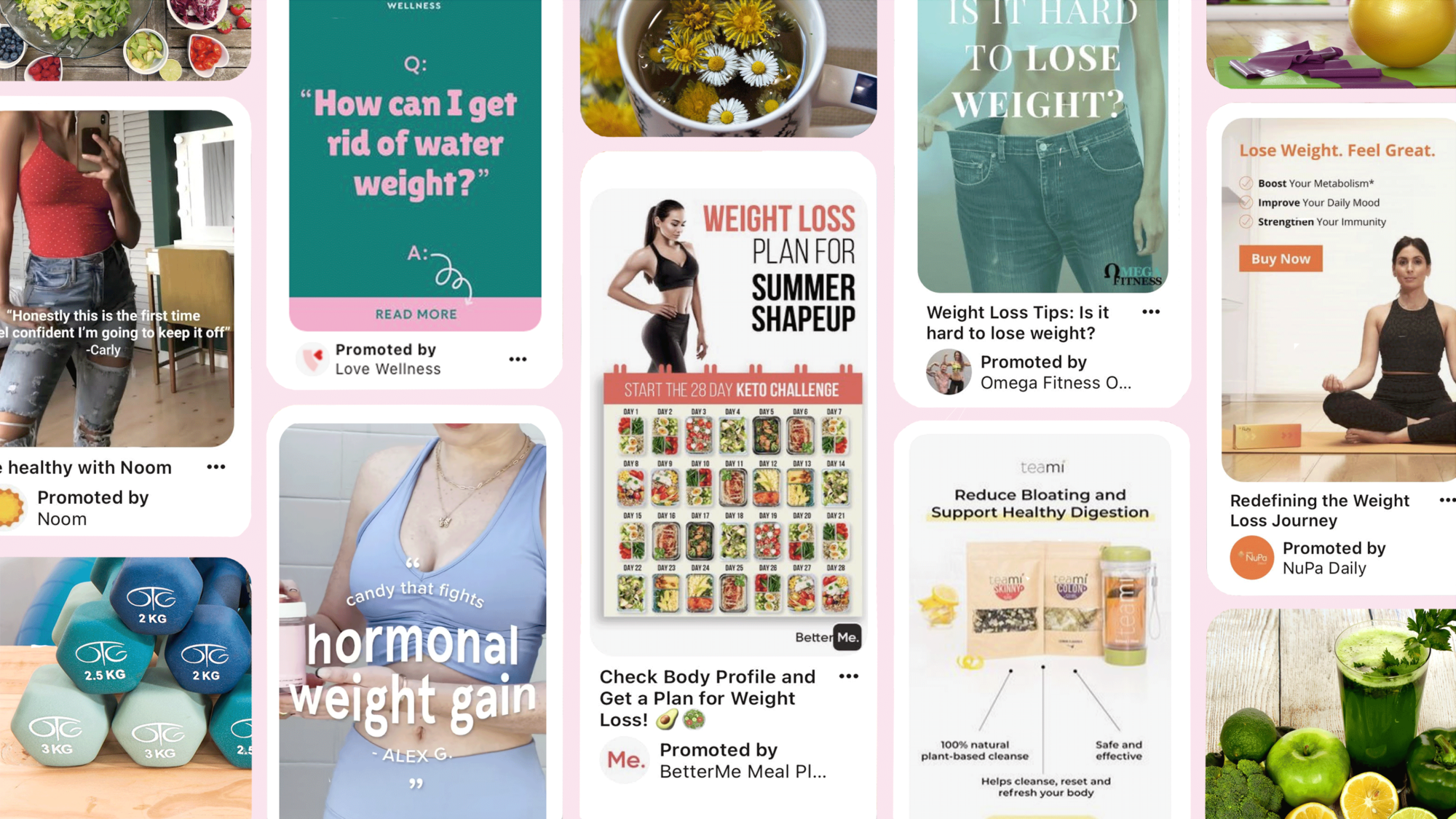
TINA’s Take: Will Pinterest Enforce Its Ban on ALL Weight-Loss Ads?
Despite the splashy announcement, weight-loss ads persist on social media platform.
TINA.org agrees with health groups that Juul's current campaign disseminates an illegal smoking cessation claim.
WHAT’S UP
A multimillion-dollar advertising campaign for Juul e-cigarettes that encourages smokers to “make the switch” from cigarettes to Juul is drawing criticism from public health advocates who say the tagline amounts to an unapproved, and therefore illegal, smoking cessation claim.
“Juul, a product that FDA has found to be largely responsible for the current epidemic of youth usage of highly addictive e-cigarettes, is being advertised and marketed on a massive scale as a smoking cessation product, without the required review and approval by FDA,” says a letter sent to the FDA earlier this month by the Campaign for Tobacco-Free Kids, the American Academy of Pediatrics, the American Cancer Society Cancer Action Network, the American Heart Association, the American Lung Association and the Truth Initiative.
It is a violation of federal law to market a product as a smoking cessation device without the review and approval of the FDA and while the agency has granted approval to a few products, including nicotine-containing gums, lozenges and patches, in addition to two prescription drugs that do not contain nicotine, it hasn’t approved any e-cigarette for the purpose of helping smokers quit.
The coalition of public health groups — which minus the American Academy of Pediatrics is the same one that wrote to the FDA in October 2015 urging the agency to investigate smoking cessation claims in a database compiled by TINA.org — called on the FDA to open an investigation and take enforcement action.
HOW WE GOT HERE
The health groups’ letter comes as the FDA is already probing whether Juul played an active role in the rapid rise of e-cigarette use among teens by intentionally marketing its devices to youth. The current marketing campaign marks a shift from the company’s earlier campaigns, which featured young models and colorful graphics. The people in today’s Juul ads who have “made the switch” are middle-aged and the ads themselves are more sober-looking.
THE MARKETING PITCH IN QUESTION
Take this black-and-white print ad that ran in the San Francisco Chronicle last December:
The ad is “Exhibit 1” in the health groups’ letter.
“The ad clearly communicates the message that by switching to Juul, the smoker can achieve what he/she has previously been unable to achieve: quitting smoking,” the letter says. “It is, unmistakably, a smoking cessation claim for Juul.”
Yet Juul does not equate switching to quitting. Its website carries the disclaimer: “Juul is a switching product. Juul products are not intended to be used as cessation products, including for the cure or treatment of nicotine addition (e.g., smoking cessation), relapse prevention, or relief of nicotine withdrawal symptoms.”
At the same time, Juul is funding research in an apparent effort to prove that its products help smokers quit. A recent survey found that nearly 50 percent of cigarette smokers who tried Juul kicked the habit within three months, though in addition to being company-funded, the survey relied on self-reported data that are susceptible to response bias.
Moreover, the Center on Addiction warns:
While a few studies have found that e-cigarettes can help reduce smoking, most show that e-cigarette use does not significantly reduce cigarette use, and several found that people who use e-cigarettes may be less likely to successfully quit smoking. This may be because e-cigarette use can perpetuate nicotine addiction.
It’s an important reminder that the science is still out on e-cigarettes and while the FDA has acknowledged the potential of e-cigarettes to offer adult smokers a possible safer alternative to combustible tobacco products that contain harmful chemicals, more research is needed.
WHAT’S NEXT
In response to a media inquiry by TINA.org, FDA spokesman Michael Felberbaum reiterated that “the FDA has not approved any e-cigarette product as a smoking cessation aid under the safety and efficacy standard governing FDA-regulated medical products.”
It’s the FDA’s job to regulate e-cigarettes and Juul makes up 75 percent of the market — a portion that may grow even larger now that the company has the backing of tobacco giant Altria, maker of Marlboro cigarettes.
In addition to print, Juul’s “make the switch” ads appear on TV and radio, both mediums where advertising of traditional cigarettes is banned.
TINA.org agrees that Juul’s current marketing campaign disseminates an illegal smoking cessation claim and echoes the health groups’ call for the FDA to open an investigation and take enforcement action.
Find more of our coverage on the FDA’s efforts to rein in Juul’s marketing here.
Despite the splashy announcement, weight-loss ads persist on social media platform.
FDA says it is aware of the situation but declined to comment on the regulatory status of disposable e-cigarette brand.
The disposable e-cigarette has replaced Juul as the go-to vape for minors.