Puff Bar Flavors Resurface Following FDA Ban
FDA says it is aware of the situation but declined to comment on the regulatory status of disposable e-cigarette brand.
One of the brands whose sales were halted is popular among youth smokers.
The FDA has halted the sales of four cigarette brands including Camel Crush Bold, which is a popular brand among youth smokers, marking the second time this summer that the agency has exercised its authority under a far-reaching 2009 tobacco-control law to regulate the industry.
The agency said it ordered the four brands, which carried new features such as a higher level of menthol, off the market because R.J. Reynolds, the second-largest tobacco company in the country, failed to prove that they do not present any more health risks than products previously sold.
“These decisions were based on a rigorous, science-based review designed to protect the public from the harms caused by tobacco use,” said Mitch Zeller, director of the FDA’s Center for Tobacco Products.
The order signals that the FDA is stepping up its regulatory muscle under the Tobacco Control Act of 2009 to pursue action against cigarette companies. In August, the agency warned the makers of Winston, Natural American Spirit and Nat Sherman cigarettes that advertising their smokes as “natural” and/or “additive-free” violates the tobacco-control law. The FDA said the companies did not have its approval to market their cigarettes with claims that the agency said implied they are safer than other brands.
The Campaign for Tobacco-Free Kids applauded the FDA’s efforts to order a major cigarette brand — Camel Crush Bold — off the market. Matthew Myers, the group’s president, said:
Before the 2009 law, tobacco companies were free to change their products in secret, and no government agency had the authority to do anything about it. The FDA now has the authority to stop these harmful tobacco industry actions, and the agency’s action today is a much-needed step forward.
The other three products ordered pulled from the shelves were Pall Mall Deep Set Recessed Filter, Pall Mall Deep Set Recessed Filter Menthol and Vantage Tech 13.
Find more of TINA.org’s coverage on tobacco here.
FDA says it is aware of the situation but declined to comment on the regulatory status of disposable e-cigarette brand.
TINA.org agrees with health groups that Juul’s current campaign disseminates an illegal smoking cessation claim.
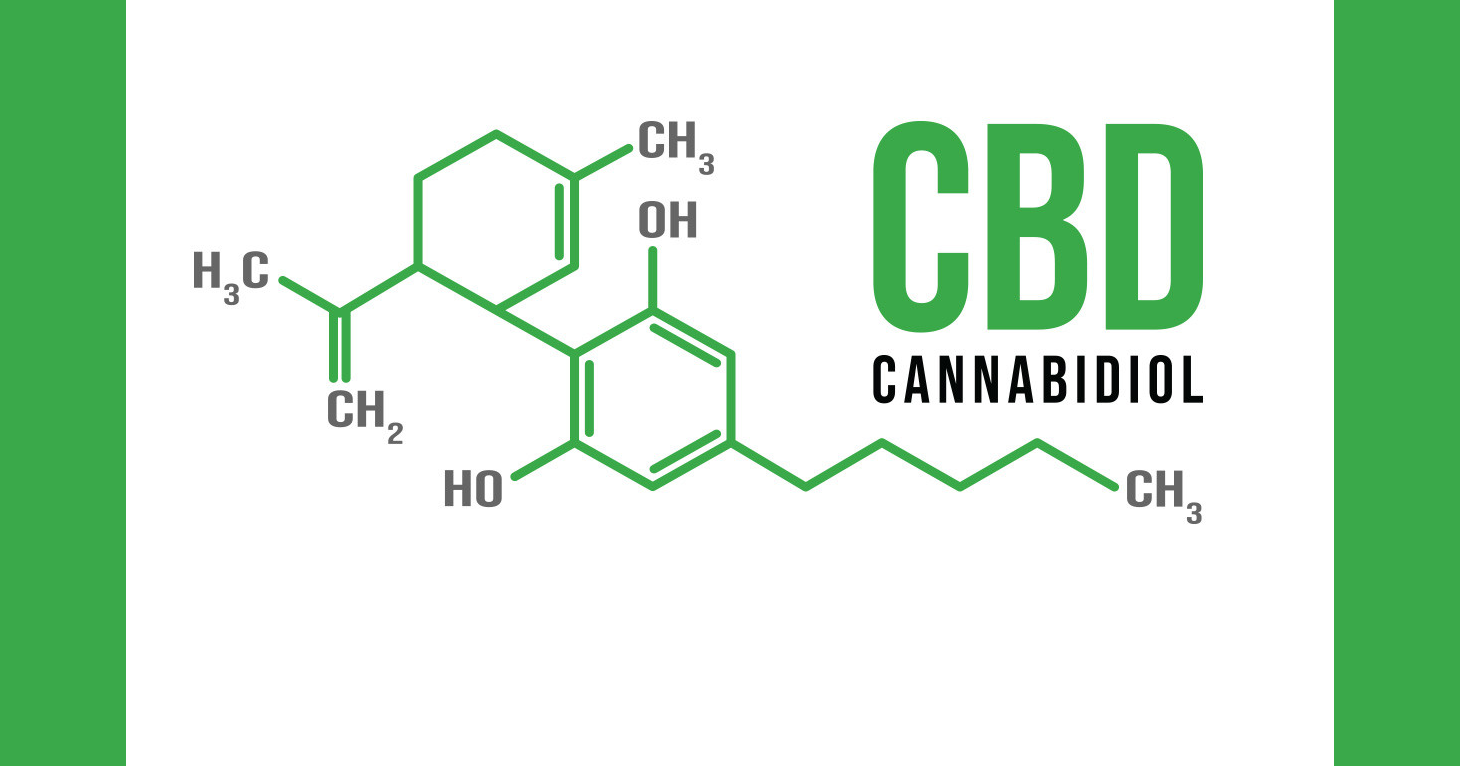
FDA to consider legal pathways for cannabis-derived compound.