Sudafed, Tylenol, DayQuil, NyQuil and Store Brand Cold and Flu Medicines
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Diesel et al. v. The Procter & Gamble Co.
22-cv-892, E.D. Mo.
(July 2022)
Meza et al. v. The Procter & Gamble Co.
23-cv-241, S.D. Ohio
(Jan. 2023)
DayQuil cough medicine
Falsely marketing medicine as “non-drowsy” when an ingredient (dextromethorphan hydrobromide, or DXM) causes drowsiness
Pending
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Misleadingly marketing products as if they alleviate cold and flu symptoms when fine print on the side of the product packaging discloses that the products are “not intended to…
Allegations: Misleadingly marketing medicines as “non-drowsy” when an ingredient in them causes drowsiness
TINA.org found the German automaker didn’t have the support to claim its Sprinter van was “built in the USA.”
These brand-relationship disclosures are far from world-class.
Instant replay shows six instances in which Super Bowl 52 advertisers have fumbled ad claims.
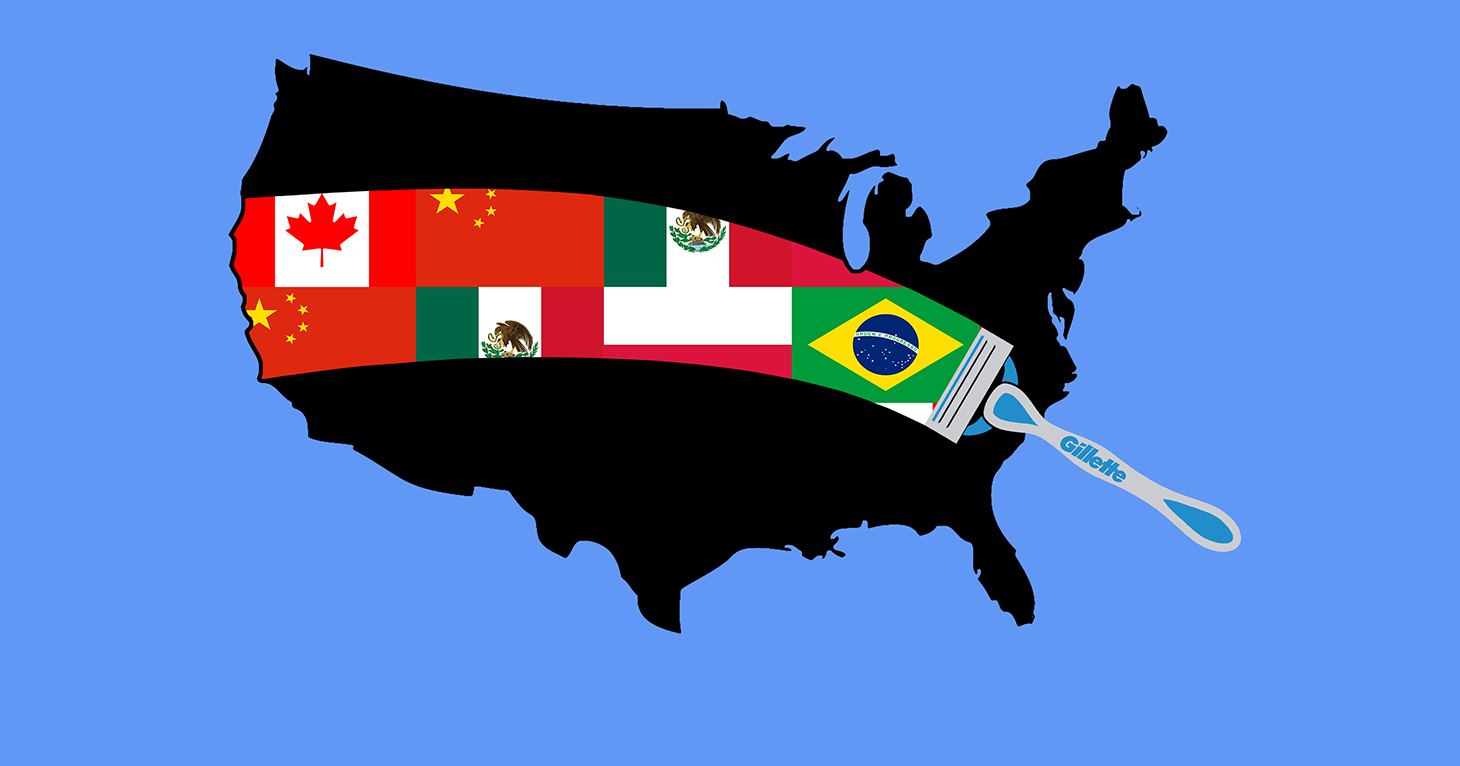
Ad Watchdog TINA.org Files FTC Complaint MADISON, CONN. January 23, 2018 – Despite a national advertising campaign showcasing patriotic imagery, highlighting its Boston headquarters, and heavily promoting its “Made in…
TINA.org files FTC complaint over Gillette’s false and deceptive Made in the USA claims.