CVS
TINA.org investigated the marketing used to promote life’sDHA™, a supplement sold by CVS Pharmacy under the name Algal-900 DHA, and found that the marketing deceptively implied the product could improve memory…
April 2017: This case was transferred to a court in Maryland. (Palmer et al v. CVS Health et al, Case No. 17-cv-938, D. MD.)
May 2015: A class-action lawsuit was filed against CVS Health and Nice-Pak Products, Inc. for allegedly misleadingly marketing flushable wipes – including CVS/pharmacy® Flushable Cleansing Wipes and Total Home by CVS Flushable Wipes – as “flushable” when the wipes actually cause many plumbing problems, such as clogged toilets and pipes. (Palmer et al v. CVS Health and Nice-Pak Products, Inc., Case No. 15-cv-2928, E. D. NY.)
For more information about other class-action lawsuits regarding flushable wipes and TINA.org’s coverage of them, click here.
TINA.org investigated the marketing used to promote life’sDHA™, a supplement sold by CVS Pharmacy under the name Algal-900 DHA, and found that the marketing deceptively implied the product could improve memory…
Allegations: Falsely marketing that products contain “No Artificial Preservatives”
Allegations: Falsely marketing that products treat nail fungus
Allegations: Misleadingly marketing CVS Health Toddler Beginnings
Allegations: Falsely advertising the accuracy of ovulation test kits
Allegations: Falsely marketing products as “non habit-forming”
Allegations: Failing to disclose that products contain harmful synthetic chemicals known as PFAS
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Falsely marketing products as covered in yogurt and healthy when the coating is actually “candy-like” and contains several unhealthy ingredients
Allegations: Misleadingly representing that products were safe and tested by dermatologists when they contain, or were at risk of containing, the carcinogen benzene
Allegations: Marketing products as sterile and safe when they have been contaminated with bacteria that may cause eye infections that can lead to partial vision loss or blindness
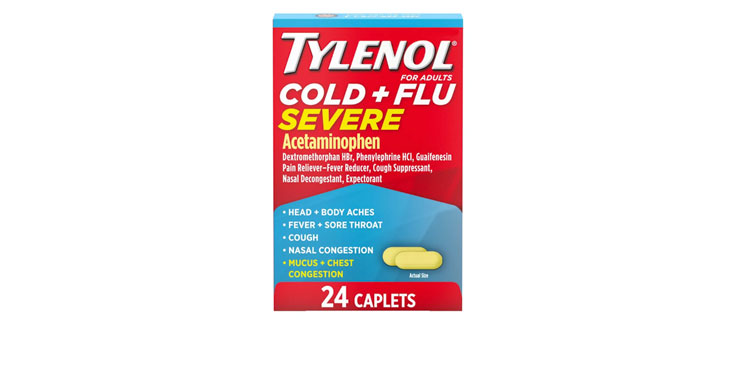
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
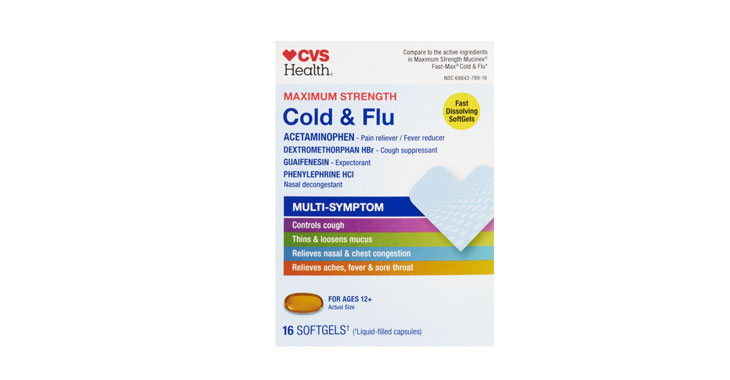
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Defrauding consumers by asking them to make donations to the American Diabetes Association on checkout screens when all or part of the donated money went to CVS
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing products as decongestants
Allegations: Falsely marketing that products treat symptoms of pink eye
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines treat congestion and other sinus issues
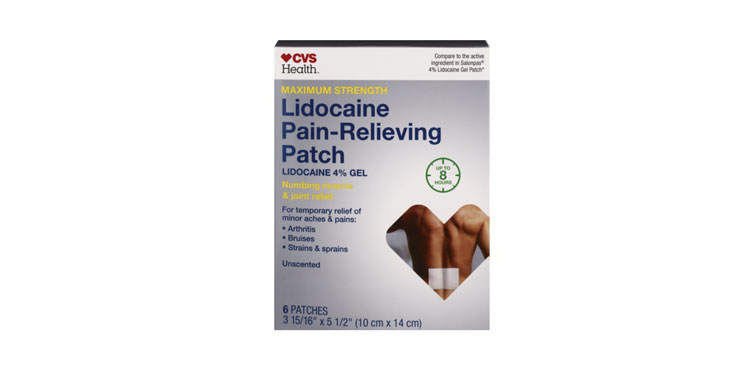
Allegations: Misleadingly marketing that patches provide “Maximum Strength” doses of lidocaine and up to 8 or 12 hours of pain relief
Allegations: Marketing that products promote a healthy mouth when using them contributes to various oral health issues
Allegations: Making misleading claims about the ingredients and capabilities of the toothpaste
Allegations: Falsely marketing products as if they repair gums and reverse gingivitis when the active ingredient does not provide such benefits
Allegations: Marketing products as safe pain relievers for pregnant women without warning consumers that scientific evidence shows prenatal exposure to APAP can cause neurodevelopmental disorders in children
Allegations: Falsely marketing hydrogen peroxide as a treatment for minor cuts and abrasions
Allegations: Falsely marketing medicine as “non-drowsy” when an ingredient causes drowsiness
Allegations: Deceiving consumers about the proper use for its cotton swabs and failing to adequately disclose the risks associated with using cotton swabs to clean in the ear canal
Allegations: Failing to disclose that products may contain a dangerous substance that increases the risk of serious adverse health consequences and death
Allegations: Failing to disclose that products contain a harmful substance and may increase the risk of contracting invasive infections
Allegations: Falsely marketing that hand sanitizers “kill[] 99.99% of germs” when scientific evidence shows that alcohol-based hand sanitizers do not kill many types of germs
Allegations: Falsely marketing that products contain more protein than they actually do
Allegations: Failing to disclose products contain the carcinogenic chemical benzene
Allegations: Misleadingly marketing products as “maximum strength” when similar products from competitors contain more lidocaine
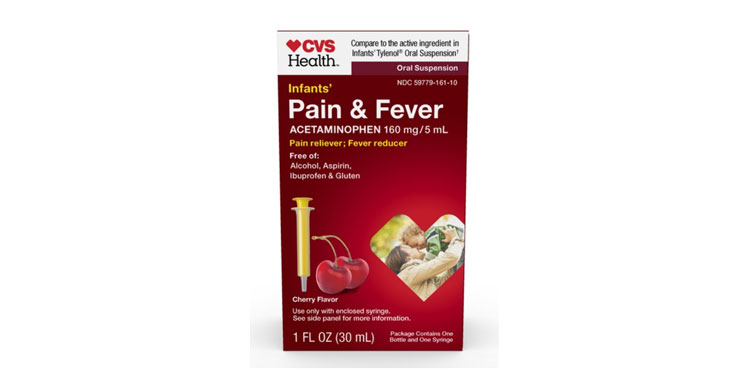
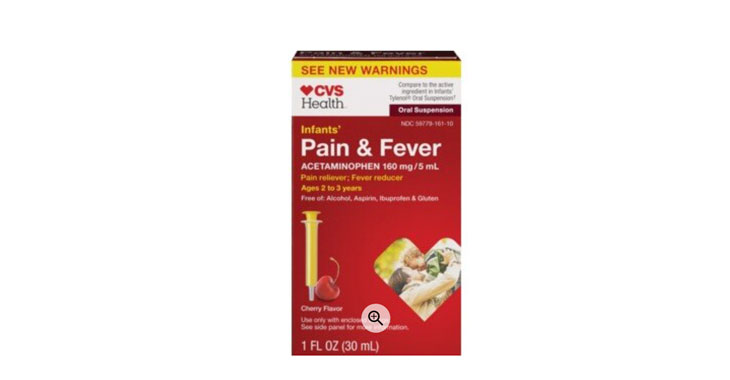
Allegations: Misleadingly marketing the infants’ product as specifically formulated for infants when it has the same concentration of acetaminophen as the children’s version
Allegations: Falsely marketing medicines as “non-drowsy” when the active ingredient in them causes drowsiness
Allegations: Misleadingly marketing that the active ingredients in sunscreens are zinc oxide minerals when there are also chemical active ingredients in the sunscreens
Allegations: Misleadingly marketing that the product is specifically formulated for infants and charging more for the infants’ product when it contains the same formulation of the same active ingredient in…
Allegations: Misleadingly marketing that hand sanitizers “Kill[] 99.99% of Germs” when there is no scientific evidence to support such claims
Several products marketed as ‘non-drowsy’ contain an ingredient that causes drowsiness, lawsuits claim.
Several store-brand aloe vera gels lack the acclaimed soothing ingredient.
TINA.org finds that the marketing of Algal-900 DHA violates FTC order.
CVS brand of memory supplement under legal fire.
Unproven cold prevention and treatment claims are nothing to sneeze at.