CVS
TINA.org investigated the marketing used to promote life’sDHA™, a supplement sold by CVS Pharmacy under the name Algal-900 DHA, and found that the marketing deceptively implied the product could improve memory…
June 2018: This action was voluntarily dismissed When a complaint is dismissed without prejudice, an amended version of the complaint can be refiled..
May 2018: A magistrate judge concluded that the named plaintiff did not demonstrate that he would fairly and adequately protect the interests of the class and recommended that the motion for preliminary approval be denied.
January 2018: CVS requested preliminary approval of a second amended settlement agreement reached between the parties. The new agreement does not amend the relief provisions but narrows the release provisions and makes some changes to the notice provisions. To read the letter and learn more about the changes to the agreement, click here.
September 2016: A federal judge granted TINA.org’s Motion for leave to file the second letter as (Latin for “friend of the courts.”) A person or organization that is not a party to a lawsuit but has a significant interest in the case and offers information that may be important to the court’s determination..
August 2016: TINA.org files a second letter as amicus curiae opposing the amended settlement agreement.
June 2016: TINA.org filed a letter as (Latin for “friend of the courts.”) A person or organization that is not a party to a lawsuit but has a significant interest in the case and offers information that may be important to the court’s determination. opposing the proposed settlement. To learn more about TINA.org’s involvement in the case, click here.
Later in June, the parties amended the settlement agreement. This agreement addresses many – but not all – of the issues raised by TINA.org regarding the injunctive relief provided in the settlement.
May 2016: After the lawsuit was transferred to a court in New York, plaintiffs moved for preliminary approval of a settlement of this lawsuit. According to the proposed settlement terms, class members with proof of purchase will receive refunds, while class members without proof of purchase will either receive $4 in cash or a $6.50 voucher to use toward a purchase of a CVS-branded dietary supplement. The company also agreed to stop using the statements “clinically shown to improve memory” or “clinically shown memory improvement” on the product label unless such claims are supported by adequate scientific evidence, as well as a vague reference to “claims at issue in this Action.” (Aliano et al v. CVS Pharmacy, Inc., Case No. 16-cv-2624, E.D.N.Y.)
February 2016: A false advertising class-action lawsuit was filed against CVS over its allegedly deceptive marketing of the supplement Algal-900 DHA as “clinically shown” to improve memory and prevent cognitive decline without having adequate substantiation to support such claims. (Aliano et al v. CVS Pharmacy, Inc., Case No. 16-cv-2624, N.D. Ill.)
For more information about other class-action lawsuits filed against CVS and TINA.org’s coverage of the company, click here.
For more information about other class-action lawsuits about products claiming to improve brain and memory function and TINA.org’s coverage of the topic, click here.
TINA.org investigated the marketing used to promote life’sDHA™, a supplement sold by CVS Pharmacy under the name Algal-900 DHA, and found that the marketing deceptively implied the product could improve memory…
Allegations: Falsely marketing products as “non habit-forming”
Allegations: Failing to disclose that products contain harmful synthetic chemicals known as PFAS
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Falsely marketing products as covered in yogurt and healthy when the coating is actually “candy-like” and contains several unhealthy ingredients
Allegations: Falsely advertising the accuracy of ovulation test kits
Allegations: Misleadingly representing that products were safe and tested by dermatologists when they contain, or were at risk of containing, the carcinogen benzene
Allegations: Marketing products as sterile and safe when they have been contaminated with bacteria that may cause eye infections that can lead to partial vision loss or blindness
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Defrauding consumers by asking them to make donations to the American Diabetes Association on checkout screens when all or part of the donated money went to CVS
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing products as decongestants
Allegations: Falsely marketing that products treat symptoms of pink eye
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines treat congestion and other sinus issues
Allegations: Misleadingly marketing that patches provide “Maximum Strength” doses of lidocaine and up to 8 or 12 hours of pain relief
Allegations: Marketing that products promote a healthy mouth when using them contributes to various oral health issues
Allegations: Making misleading claims about the ingredients and capabilities of the toothpaste
Allegations: Falsely marketing products as if they repair gums and reverse gingivitis when the active ingredient does not provide such benefits
Allegations: Misleadingly marketing CVS Health Toddler Beginnings
Allegations: Marketing products as safe pain relievers for pregnant women without warning consumers that scientific evidence shows prenatal exposure to APAP can cause neurodevelopmental disorders in children
Allegations: Falsely marketing hydrogen peroxide as a treatment for minor cuts and abrasions
Allegations: Falsely marketing medicine as “non-drowsy” when an ingredient causes drowsiness
Allegations: Deceiving consumers about the proper use for its cotton swabs and failing to adequately disclose the risks associated with using cotton swabs to clean in the ear canal
Allegations: Failing to disclose that products may contain a dangerous substance that increases the risk of serious adverse health consequences and death
Allegations: Failing to disclose that products contain a harmful substance and may increase the risk of contracting invasive infections
Allegations: Falsely marketing that hand sanitizers “kill[] 99.99% of germs” when scientific evidence shows that alcohol-based hand sanitizers do not kill many types of germs
Allegations: Falsely marketing that products contain more protein than they actually do
Allegations: Failing to disclose products contain the carcinogenic chemical benzene
Allegations: Misleadingly marketing products as “maximum strength” when similar products from competitors contain more lidocaine
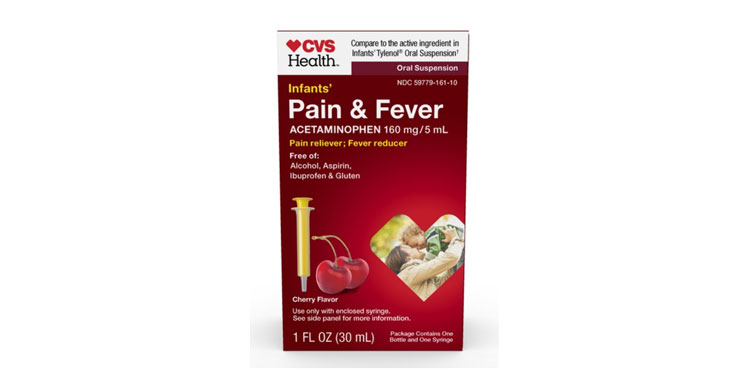
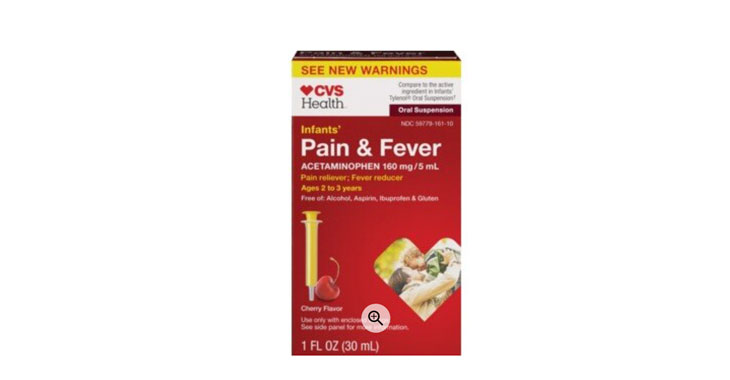
Allegations: Misleadingly marketing the infants’ product as specifically formulated for infants when it has the same concentration of acetaminophen as the children’s version
Allegations: Falsely marketing medicines as “non-drowsy” when the active ingredient in them causes drowsiness
Allegations: Misleadingly marketing that the active ingredients in sunscreens are zinc oxide minerals when there are also chemical active ingredients in the sunscreens
Allegations: Misleadingly marketing that the product is specifically formulated for infants and charging more for the infants’ product when it contains the same formulation of the same active ingredient in…
Allegations: Misleadingly marketing that hand sanitizers “Kill[] 99.99% of Germs” when there is no scientific evidence to support such claims
In November 2020, a class-action lawsuit was filed against CVS Health Corp. and Nice-Pak Products for allegedly falsely marketing wipes – including CVS Health Flushable Cleansing Wipes and CVS Health…
In July 2020, a class-action lawsuit was filed against Vi-Jon, Inc. for allegedly falsely advertising that germ-X and several store-brand hand sanitizers – including CVS Health, equate (Walmart), and Walgreens…
March 2020: The Seventh Circuit Court of Appeals affirmed the district court’s decision to grant summary judgment in favor of the companies. April 2019: A federal judge granted judgment in…
June 2019: The named plaintiff filed a Notice of Appeal regarding the dismissal order. (Case No. 19-55671, 9th Cir.) May 2019: A federal judge dismissed this case finding that the…
September 2019: A federal judge granted final approval of the settlement agreement. May 2019: A federal judge preliminarily approved a settlement agreement. According to its terms, class members who have…
In July 2018, a state judge preliminarily approved a settlement of a class-action lawsuit filed against Lang Pharma Nutrition (a company that supplies retailers with CoQ-10 supplements to sell under…
May 2015: This lawsuit was dismissed for lack of subject-matter jurisdiction. March 2015: A class-action lawsuit was filed against CVS Pharmacy and Lang Pharma Nutrition for allegedly falsely advertising CVS…
In October 2018, a class-action lawsuit was filed against Westminster Pharmaceuticals and CVS Pharmacy for allegedly representing that its Thyroid Tablets – a brand of generic prescription thyroid medication –…
April 2018: A federal judge dismissed this case because the parties reported that they reached a settlement agreement. November 2017: A class-action lawsuit was filed against CVS for allegedly deceptively…
In July 2018, a class-action lawsuit was filed against CVS for allegedly marketing the supplement Algal-900 DHA as being clinically shown to improve memory and cognitive function and claiming the…
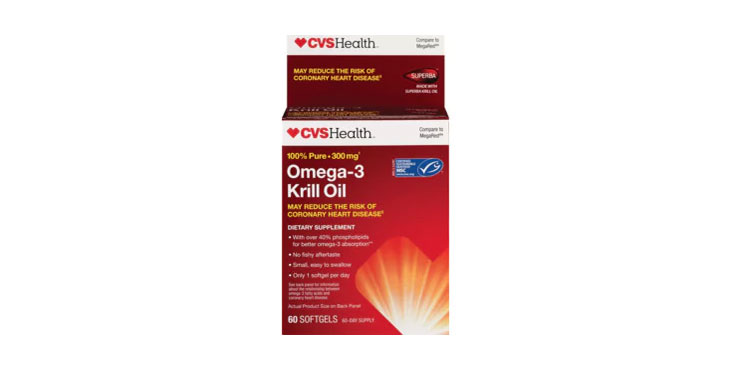
In March 2018, a class-action lawsuit was filed against Lang Pharma Nutrition and CVS Pharmacy for allegedly misrepresenting that CVS brand 100% Pure Omega-3 Krill Oil contains 300 mg of…
In December 2017, a class-action lawsuit was filed against several manufacturers of flushable wipes – specifically, Costco, CVS, Kimberly-Clark, Procter & Gamble, Target, Walgreens, and Walmart – for allegedly falsely…
In November 2017, a class-action lawsuit was filed against CVS for allegedly falsely marketing the SPF in CVS Sport sunscreens as 100+ when plaintiffs claim that the actual SPF of…
May 2017: This action was voluntarily dismissed When a complaint is dismissed with prejudice, it cannot be refiled., the reasons for which have not been disclosed. September 2016: The First…
In August 2017, a false advertising class-action lawsuit was filed against CVS. Among other things, plaintiffs allege that CVS deceptively represents that it provides customers with more affordable prescription drugs…
April 2017: This case was transferred to a court in Maryland. (Palmer et al v. CVS Health et al, Case No. 17-cv-938, D. MD.) May 2015: A class-action lawsuit was…
A false advertising class-action lawsuit against CVS survived a motion to dismiss filed by CVS in March 2016. The complaint, which was originally filed in November 2014 and amended in…
August 2015: A federal judge dismissed this action When a complaint is dismissed without prejudice, an amended version of the complaint can be refiled. for lack of subject matter jurisdiction.…
March 2016: This lawsuit was dismissed When a complaint is dismissed with prejudice, it cannot be refiled. in accordance with the terms of a confidential settlement agreement. December 2015: A…
In February 2016, a class-action lawsuit was filed against CVS for allegedly deceptively representing that CVS Flu Relief “[t]emporarily relieves flu-like symptoms” and “reduces the duration & severity of flu-like…
These definitions are a joke.
An FDA panel’s recent findings has led to a flood of lawsuits.
This ad hits different when you read the fine print.
FDA targets companies selling eye drops illegally marketed to treat conditions like pink eye.
Plaintiffs allege packaging misrepresents lidocaine dosages as ‘maximum strength,’ among other things.