CVS
TINA.org investigated the marketing used to promote life’sDHA™, a supplement sold by CVS Pharmacy under the name Algal-900 DHA, and found that the marketing deceptively implied the product could improve memory…
March 2020: The Seventh Circuit Court of Appeals affirmed the district court’s decision to grant summary judgment in favor of the companies.
April 2019: A federal judge granted judgment in favor of CVS, Target, and Walmart in order to be consistent with the previous summary judgment ruling in favor of the other defendants. Later in the month, plaintiffs filed a Notice of Appeal regarding the March 2019 summary judgment order.
March 2019: A federal judge granted a motion for summary judgment filed by Fruit of the Earth and Walgreens concluding that the label of the Fruit of the Earth gel is not misleading and plaintiffs did not provide enough evidence to show that the products do not provide sunburn relief.
2017: The claims of several plaintiffs were voluntarily dismissed When a complaint is dismissed without prejudice, an amended version of the complaint can be refiled. and the name of the case was changed to Beardsall v. CVS.
Also in 2017, plaintiffs filed an amended complaint adding Target, Walgreens, Walmart, and Fruit of the Earth as defendants. This complaint alleges that the retailers and marketers misleadingly represent that products contain aloe vera and provide consumers with the healing and restorative benefits of aloe when, according to plaintiffs, the products do not contain detectible amounts of aloe vera.
June 2016: A class-action lawsuit was filed against CVS for, among other things, allegedly falsely labeling CVS 100% Pure Aloe Vera Gel as containing “100% pure aloe vera gel” when, according to plaintiffs, the product contains no aloe at all. (Bordenet et al v. CVS Health Corp., Case No. 16-cv-6103, N. D. Ill.)
For more information about other class-action lawsuits filed against CVS and TINA.org’s coverage of the company, click here.
TINA.org investigated the marketing used to promote life’sDHA™, a supplement sold by CVS Pharmacy under the name Algal-900 DHA, and found that the marketing deceptively implied the product could improve memory…
Allegations: Falsely marketing that products contain “No Artificial Preservatives”
Allegations: Falsely marketing that products treat nail fungus
Allegations: Misleadingly marketing CVS Health Toddler Beginnings
Allegations: Falsely advertising the accuracy of ovulation test kits
Allegations: Falsely marketing products as “non habit-forming”
Allegations: Failing to disclose that products contain harmful synthetic chemicals known as PFAS
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Falsely marketing products as covered in yogurt and healthy when the coating is actually “candy-like” and contains several unhealthy ingredients
Allegations: Misleadingly representing that products were safe and tested by dermatologists when they contain, or were at risk of containing, the carcinogen benzene
Allegations: Marketing products as sterile and safe when they have been contaminated with bacteria that may cause eye infections that can lead to partial vision loss or blindness
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Defrauding consumers by asking them to make donations to the American Diabetes Association on checkout screens when all or part of the donated money went to CVS
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing products as decongestants
Allegations: Falsely marketing that products treat symptoms of pink eye
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines treat congestion and other sinus issues
Allegations: Misleadingly marketing that patches provide “Maximum Strength” doses of lidocaine and up to 8 or 12 hours of pain relief
Allegations: Marketing that products promote a healthy mouth when using them contributes to various oral health issues
Allegations: Making misleading claims about the ingredients and capabilities of the toothpaste
Allegations: Falsely marketing products as if they repair gums and reverse gingivitis when the active ingredient does not provide such benefits
Allegations: Marketing products as safe pain relievers for pregnant women without warning consumers that scientific evidence shows prenatal exposure to APAP can cause neurodevelopmental disorders in children
Allegations: Falsely marketing hydrogen peroxide as a treatment for minor cuts and abrasions
Allegations: Falsely marketing medicine as “non-drowsy” when an ingredient causes drowsiness
Allegations: Deceiving consumers about the proper use for its cotton swabs and failing to adequately disclose the risks associated with using cotton swabs to clean in the ear canal
Allegations: Failing to disclose that products may contain a dangerous substance that increases the risk of serious adverse health consequences and death
Allegations: Failing to disclose that products contain a harmful substance and may increase the risk of contracting invasive infections
Allegations: Falsely marketing that hand sanitizers “kill[] 99.99% of germs” when scientific evidence shows that alcohol-based hand sanitizers do not kill many types of germs
Allegations: Falsely marketing that products contain more protein than they actually do
Allegations: Failing to disclose products contain the carcinogenic chemical benzene
Allegations: Misleadingly marketing products as “maximum strength” when similar products from competitors contain more lidocaine
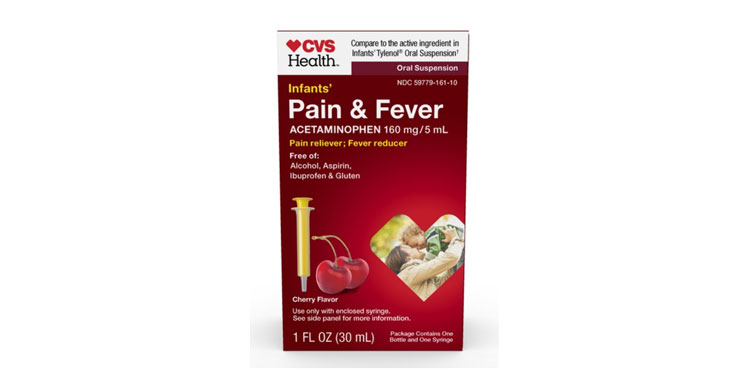
Allegations: Misleadingly marketing the infants’ product as specifically formulated for infants when it has the same concentration of acetaminophen as the children’s version
Allegations: Falsely marketing medicines as “non-drowsy” when the active ingredient in them causes drowsiness
Allegations: Misleadingly marketing that the active ingredients in sunscreens are zinc oxide minerals when there are also chemical active ingredients in the sunscreens
Allegations: Misleadingly marketing that the product is specifically formulated for infants and charging more for the infants’ product when it contains the same formulation of the same active ingredient in…
Allegations: Misleadingly marketing that hand sanitizers “Kill[] 99.99% of Germs” when there is no scientific evidence to support such claims
These definitions are a joke.
An FDA panel’s recent findings has led to a flood of lawsuits.
This ad hits different when you read the fine print.
FDA targets companies selling eye drops illegally marketed to treat conditions like pink eye.
Plaintiffs allege packaging misrepresents lidocaine dosages as ‘maximum strength,’ among other things.