ChapStick Natural Claims
Allegations: False natural claims
March 2016: A federal judge denied plaintiff’s request for permission to file a second amended complaint finding that the amended complaint would not survive a motion to dismiss and, as a result, is futile.
March 2015: A federal judge dismissed this class-action lawsuit because the plaintiffs did not sufficiently plead that the company deceptively marketed the product. Among other things, the judge found that “the science does not undercut Centrum’s statements regarding its health benefits” for two reasons: First, the plaintiffs cite scientific materials that do not address the same health issues as the ones in Centrum’s marketing; and second, the plaintiffs cite scientific studies that do not address the health benefits of multivitamins. The judge gave the plaintiffs 30 days to file an amended complaint.
December 2013: A class-action lawsuit was filed against Pfizer, Inc. for allegedly falsely labeling and marketing Centrum multi-vitamins. Specifically, the complaint claims the multi-vitamins – including Centrum® Silver® Adults 50+ and Centrum® Adults – will provide positive health benefits and prevent chronic illness without scientific evidence to support such claims. (Kardovich et al v. Pfizer, Inc., Case No. 13-cv-07378, E. D. NY.).
Allegations: False natural claims
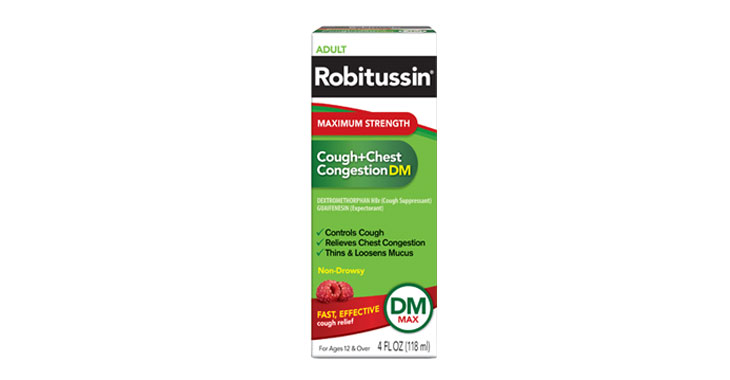
Allegations: Falsely marketing medicine as “Non-Drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing the ingredients in and the health benefits provided by products
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that medicines relieve nasal congestion and sinus pressure
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing that medicines relieve nasal congestion
Allegations: False natural claims
Allegations: Falsely marketing products as non-drowsy when an ingredient in them causes drowsiness
Allegations: Deceptively marketing products as “Maximum Strength” when the dosage of the active ingredients is less than or the same as the regular strength products
An FDA panel’s recent findings has led to a flood of lawsuits.
Several products marketed as ‘non-drowsy’ contain an ingredient that causes drowsiness, lawsuits claim.
TINA.org’s election coverage focuses on the questionable ads running in between the news broadcasts.
Is Big Pharma marketing a drug to help aging men with low sex drives or really selling a made-up disease?
Justice Department says company put profits over safety.