Byte Invisible Braces
NAD takes issue with blanket disclosure regarding incentivized reviews.
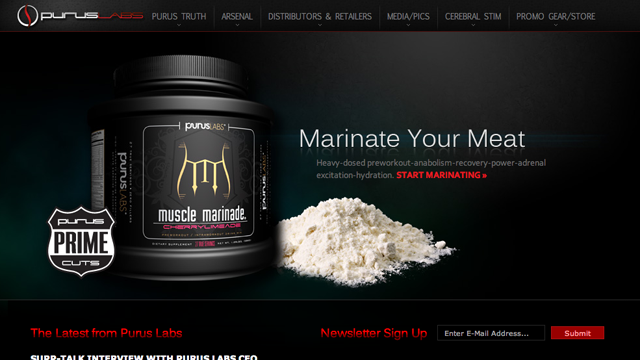
The FDA sent a warning letter to Formulife, Inc./Purus Labs, Inc., after the dietary supplement maker was found to be selling products containing DMAA, which has been banned by the FDA.
DMAA is a potentially dangerous ingredient that has been linked to health problems and deaths. Formulife and Purus Labs was selling products such as “Fat Smack™ XR Thermolipolytic”, “Muscle Marinade® Fresh Fruit”, and “Muscle Marinade® Cherry Limeade,” which all contained DMAA.
Consumers should avoid products containing DMAA. For more on this ingredient, click here.
Our Ad Alerts are not just about false and deceptive marketing issues, but may also be about ads that, although not necessarily deceptive, should be viewed with caution. Ad Alerts can also be about single issues and may not include a comprehensive list of all marketing issues relating to the brand discussed.
NAD takes issue with blanket disclosure regarding incentivized reviews.
Are there really any benefits to drinking alkaline water?
What you need to know about this purported hangover remedy.