
Most Deceptive Ads of 2024
Here were some of the worst ads TINA.org investigated this year.
Civil penalties follow a 2022 TINA.org investigation and complaint to the FTC.
|
UPDATE 1/27/23: High-ranking doTerra distributors Eliza Bacot, Lauren Busch and Dr. Tina Wong have been ordered to pay civil penalties of $15,000 each to settle Department of Justice charges they violated the FTC Act and the COVID-19 Consumer Protection Act by promoting doTerra products for the prevention and treatment of COVID-19, including during webinars (discussed below) that TINA.org alerted regulators to in January 2022.
UPDATE 2/8/22: The next presentation in the “Protocols for the Current Climate” series, originally scheduled for Feb. 16, has been canceled. Taking its place will be a presentation titled “Thinking Outside of the Marketplace,” according to an upcoming events slide shared with attendees on a Feb. 2 Zoom call. Our original article follows.
Throughout January, high-ranking distributors of essential oils Multilevel Marketing – a way of distributing products or services in which the distributors earn income from their own retail sales and from retail sales made by their direct and indirect recruits. doTerra, who are also employed or retired health care providers, have been participating in Zoom calls that have attracted hundreds of attendees. If the title of the presentations is a bit vague – “Protocols for the Current Climate” – the purpose of the calls is clear: to deceptively promote doTerra products for the treatment and prevention of COVID-19.
This comes after the FTC, in April 2020, sent doTerra a warning letter in which the federal agency cited a number of social media posts made by distributors that it said “unlawfully advertise that certain products treat or prevent COVID-19.” As an MLM responsible for the claims of its distributors, the FTC advised doTerra to immediately cease making all such claims.
With the message not received and another call planned in the coming weeks, TINA.org has alerted the FTC, among other regulators, to the ongoing deception urging prompt enforcement action. TINA.org was tipped off to the “Protocols” series by a reader.
‘COVID prevention basics’

On the first Zoom call, held on Jan. 12, Dr. Tina Wong, who introduced herself as a board-certified pediatrician, was the first of three presenters to speak. Tasked with “COVID prevention basics,” she urged everyone to “find your favorite immune oil that you have around and use it, use it frequently, use it all three ways (internally, topically and aromatically). My favorites are On Guard and oregano,” two doTerra essential oils.
Only after touting doTerra’s products for the prevention of COVID did Wong mention methods that the CDC recommends, such as washing your hands and wearing a mask. She left out getting vaccinated, which the CDC says is “the best way to slow the spread” of the virus.
Testing positive
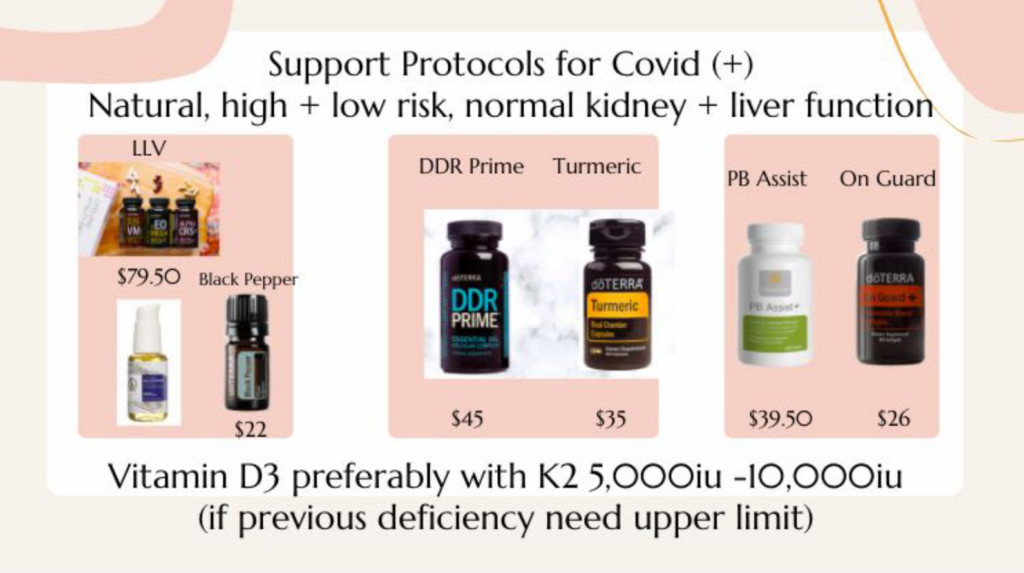
Next came Dr. Lisa Ma, who said she went to medical school with Wong and has been using doTerra products for the last eight years. Her job was to tackle what to do if you test positive for COVID-19. She started by saying, “A lot of these things that we use in doTerra do both, they will kill the virus, stop viral replication, as well as decrease inflammation.” Then, in less than a minute, she rattled off four doTerra products to take if you become infected:
Once you get COVID you want to go a little above, beyond the regular dose, which is two tablets, twice a day, of the LLV (Lifelong Vitality Pack). You can add some black pepper as well. … And then, take it to the next level, there’s the DDR Prime and the turmeric capsules. Load heavily on it. Two to four capsules within the first two hours of onset of symptoms, or right when you test positive you want to start loading up on it.
And “for the kids,” Ma recommended On Guard chewables.
Ma then cautioned against taking Tylenol or Motrin to treat a fever and instead suggested applying doTerra’s essential oils to your neck, chest and back “so you could break your fever faster.”
Also concerning was a statement by Ma that while she’s partnered with Wong for years to get the word out about the company’s essential oils and supplements, their efforts to “educate” the public went into “super drive” when the pandemic hit. Both Wong and Ma are licensed to practice medicine in the state of California. TINA.org has also alerted regulators there.
Long-haul COVID

Last to speak on the Jan. 12 call was Eliza Bacot, who was billed as an acute care nurse practitioner and who was charged with discussing how doTerra’s products help with the symptoms of long-haul COVID, such as brain fog, headache, fever, confusion, fatigue and skin rashes. The doTerra products that Bacot recommended: LLV Zendocrine Complex, DDR Prime and turmeric.
“Turmeric is very important,” Bacot said. “I would say if your symptoms are severe that you can push that turmeric dose a little bit higher.”
Taking the opportunity to plug one of her own products, Ma also claimed that a drug she invented with another doctor, called NacroPro+, has been “so helpful for some of my long-haul patients,” adding: “Even a year after long-hauling, they just take a couple packets four times a day and they are feeling better within three or four days.”
Repeat performances
The following week and the week after that saw similar performances on Zoom. On a Jan. 19 call, the host, Lauren Busch, herself a retired registered nurse, suggested the company’s Melissa Oil, a 5 ml bottle that sells for over $100, is highly effective against the coronavirus.
Joining Busch on the Jan. 19 call was Kelly Couch, a retired physician assistant and doTerra “diamond wellness advocate,” according to a post on her Facebook page. On the call, Couch said:
I assume it’s COVID until it’s proven not and I treat it, and I treat it like this: I treat it with ivermectin, I treat it with vitamin D, vitamin C, quercetin, zinc, all those things you’re already doing, all those supplements, you’re just going to increase the dose.
A week later, on a Jan. 26 call, Bacot was back, promoting, among other things, LLV Zendocrine Complex for long-haul COVID.
Of note, when registering for one of these calls, attendees must agree to a disclaimer that states:
This Zoom presentation does not constitute as medical advice and is intended for educational purposes only. Please consult your own provider and work in partnership with them to create the best treatment plan for your health.
The disclaimer adds: “Attendees are not allowed to reproduce this presentation, including sharing screenshots and photographs on social media, or any other from (sic) of reproduction of any form.”
Past inquiries
The FTC, which also sent doTerra a penalty offense notice in October 2021, isn’t the only federal agency that has called out doTerra’s deceptive marketing practices. In September 2014, the FDA sent doTerra a warning letter for, among other things, marketing its products as unapproved drugs.
The MLM has also been the subject of inquiries by the National Advertising Division, the National Advertising Review Board and the Direct Selling Self-Regulatory Council (DSSRC), which issued its first of two decisions involving doTerra after TINA.org filed a complaint with the group in October 2019. In addition to the company’s deceptive health claims, the DSSRC also took issue with its atypical income claims.
Speaking of which, on her blog, The Organic South, Bacot claims “financial fredom (sic)” is available to those who join her “business team.” And on her website, Dr. Tina’s Health Essentials, Wong promotes the business opportunity as a way to earn “residual income … while staying at home with your kids.” The reality? Most people who join legitimate MLMs make little or no money.
doTerra’s ‘leaders’
Busch, Bacot and Wong are all featured in the latest edition of doTerra’s “leadership” magazine, Busch as a platinum distributor, Bacot as a diamond distributor and Wong as achieving the rank of blue diamond. Given doTerra’s track record, it’s perhaps not surprising that the MLM would choose to honor distributors that are so reckless in the promotion of unproven remedies for COVID-19.
The consumer harm that results from health care providers recommending treatments that are not supported by competent and reliable scientific evidence cannot be overstated. The next call in the “Protocols” series is scheduled for Feb. 16. That gives regulators more than two weeks to stop it from happening.
Find more of our coverage on doTerra here.
Here were some of the worst ads TINA.org investigated this year.
Complaints against additional distributors who hosted COVID webinars may be forthcoming.
Prohibited content slips through the cracks.

