Winter Tonic Plus
The seasons change but the FDA’s position on unapproved drug treatment claims does not.
Our Ad Alerts are not just about false and deceptive marketing issues, but may also be about ads that, although not necessarily deceptive, should be viewed with caution. Ad Alerts can also be about single issues and may not include a comprehensive list of all marketing issues relating to the brand discussed.
The seasons change but the FDA’s position on unapproved drug treatment claims does not.
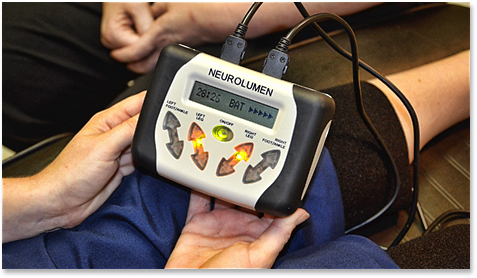
Brochure for medical device targeted military members with illegal claims to treat PTSD, among other diseases.
FDA goes after supplements making unapproved drug treatment claims.
Homeopathic spray becomes the first to adopt disclosure admitting lack of scientific evidence.
“They’re manipulating people, toying with their understanding of what contraceptive is and isn’t.”
Clinic needs to finish taking its medicine after receiving FDA warning regarding cancer treatment claims.
The breathing room for this supplement decreases as NAD refers claims to the FTC.
NAD has a bone to pick with this supplement.
The most vexing thing of all? The brain supplement admits there are no studies to back up its claims.
Discerning the difference between an antique and an antique finish.