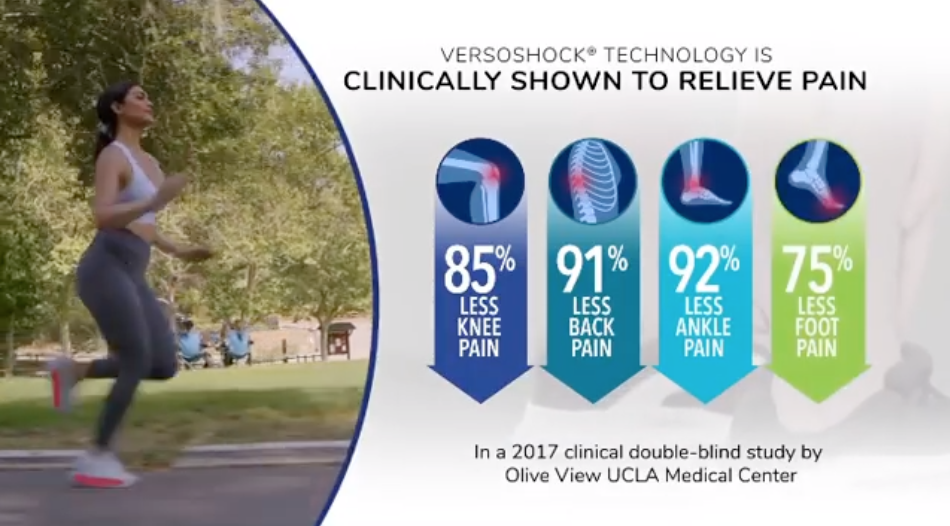
Gravity Defyer Shoes
“Clinically proven” pain relief claims come under fire.
What can these "performance pajamas" offer that a good night's sleep can't?
When Lunya gets around to fixing a typo in a blurb on its website about its new Restore collection of “smart sleepwear,” the Los Angeles-based company might also want to consider editing (or deleting) some of the bold health claims that reside therein. The blurb, brought to our attention by a reader, states:
The Restore line features Lunya’s soft and breathable Pima cotton and with Celliant® minerals. This proprietary mineral mix is the real deal; it’s regulated by the FDA, and works to absorb and convert body heat into infrared energy which is then recycled back into your skin and tissues, helping to rebuild and recharge the body during sleep. Celliant® powered fabrics also increase oxygen levels in the body which allows your cells to to regenerate faster, making it the perfect follow on for a tough workout.
Did you catch the extra “to” in the last sentence? Lunya didn’t. More importantly, what’s your take on the company’s claim that its pajamas boldly go where no pajamas have gone before, “into the skin and tissues,” to help “rebuild and recharge the body during sleep”? Here’s ours:
Find more of our coverage on sleep products here.
Our Ad Alerts are not just about false and deceptive marketing issues, but may also be about ads that, although not necessarily deceptive, should be viewed with caution. Ad Alerts can also be about single issues and may not include a comprehensive list of all marketing issues relating to the brand discussed.
“Clinically proven” pain relief claims come under fire.
Marketer makes some bold claims related to the coronavirus.
Customer testimonials aren’t a good fit for claims that shoe inserts address medical conditions like plantar fasciitis.


