ChapStick Natural Claims
Allegations: False natural claims
Bell et al. v. GlaxoSmithKline Consumer Healthcare Holdings (US) LLC et al.
21-cv-9454, C.D. Cal.
(Dec. 2021)
Calchi et al. v GlaxoSmithKline Consumer Healthcare Holdings (US) LLC et al.
22-cv-1341, S.D.N.Y.
(Feb. 2022)
Muller et al. v. GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
22-cv-670, E.D. Mo.
(May 2022)
Papalia et al. v. GlaxoSmithKline Consumer Healthcare Holdings (US) LLC
22-cv-2630, S.D.N.Y.
(March 2022)
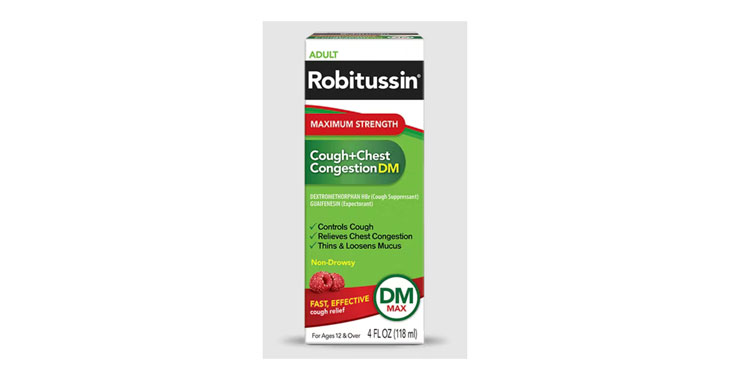
Robitussin cough medicine with dextromethorphan hydrobromide (DXM)
Falsely marketing medicine as “Non-Drowsy” when the active ingredient causes drowsiness
Bell case: Voluntarily dismissed
Calchi case: Claims against Pfizer were dismissed; Motion for preliminary approval of a settlement agreement that would resolve claims against GSK filed
Muller case: Pending
Papalia case: Consolidated with the Calchi case
Allegations: False natural claims
Allegations: Falsely marketing products as containing “Natural Flavors”
Allegations: Misleadingly marketing that products are made with natural flavors
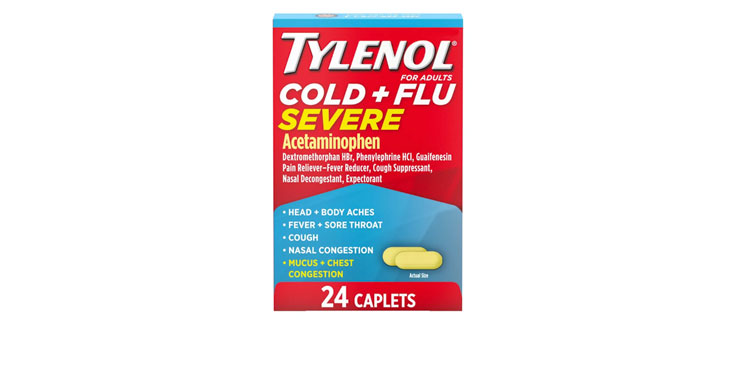
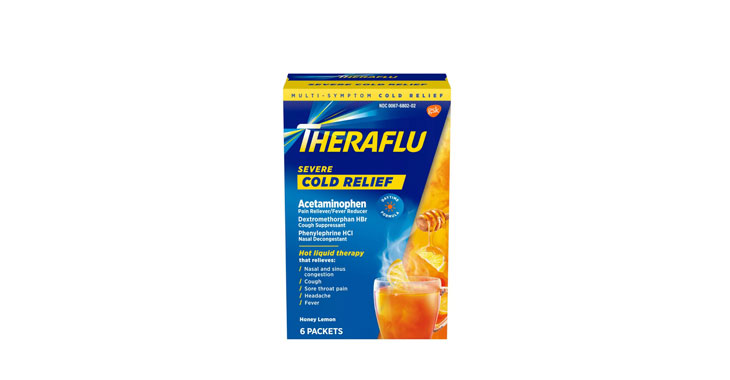
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Misleadingly marketing the ingredients in and the health benefits provided by products
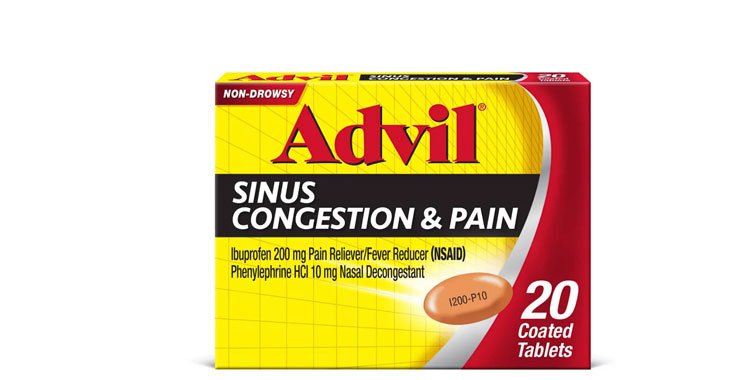
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing products as decongestants
Allegations: False natural claims
Allegations: Falsely marketing products as non-drowsy when an ingredient in them causes drowsiness
Allegations: Misleadingly marketing Abreva cold sore treatments
Several products marketed as ‘non-drowsy’ contain an ingredient that causes drowsiness, lawsuits claim.
Lip balm’s own directions seem to contradict its “8 Hour Moisture” labeling claim.
Cue the play-off music.
Is Big Pharma marketing a drug to help aging men with low sex drives or really selling a made-up disease?
GSK will pay out $105 million in off-label use case.