Walmart
TINA.org investigations into Walmart have revealed that the retail giant repeatedly engaged in false and deceptive Made in USA marketing on its website, and used undisclosed stealth marketing directed at…
April 2019: A federal judge dismissed certain claims but allowed the false advertising claims to move forward.
November 2018: A class-action lawsuit was filed against Walmart for allegedly misleadingly marketing its Equate™ Rapid Release products as being comparable to Tylenol® Extra Strength Rapid Release Gels when, according to plaintiffs, the Tylenol® products have “unique laser drilled holes” that the Equate™ products do not have. Plaintiffs also claim that Walmart misleadingly markets the Equate™ products as “rapid release” when, according to plaintiffs, the medicine does not work faster than non-rapid release products. (Plaintiffs filed an amended complaint in January 2019.) (Edwards et al v. Walmart, Inc., Case No. 18-cv-9655, C.D. Cal.)
For more information about other class-action lawsuits regarding Equate™ products and TINA.org’s coverage of them, click here.
TINA.org investigations into Walmart have revealed that the retail giant repeatedly engaged in false and deceptive Made in USA marketing on its website, and used undisclosed stealth marketing directed at…
Allegations: Falsely marketing sunscreens as “Reef Friendly”
Allegations: Falsely marketing sunscreens as “Reef Friendly”
Allegations: Misleadingly representing that products were safe when they contain, or were at risk of containing, the carcinogen benzene
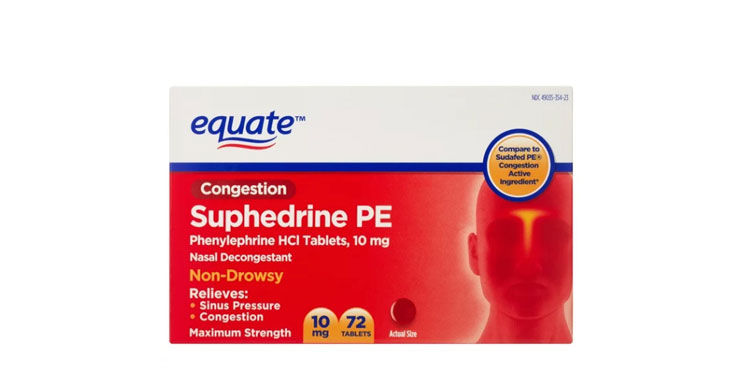
Allegations: Falsely marketing products as non-drowsy
Allegations: Falsely marketing that devices accurately measure blood pressure
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that medicines relieve nasal congestion and sinus pressure
Allegations: Falsely marketing that medicines combat congestion and other sinus issues
Allegations: Falsely marketing that medicines relieve nasal decongestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Misleadingly marketing medicines as “non-drowsy” when an ingredient in them causes drowsiness
Allegations: Falsely marketing sunscreens as “hypoallergenic” when they contain a significant amount of allergens, irritants, and other damage-causing chemicals
Allegations: Falsely marketing the product treats minor cuts and abrasions when scientific evidence shows it does not have such treatment capabilities
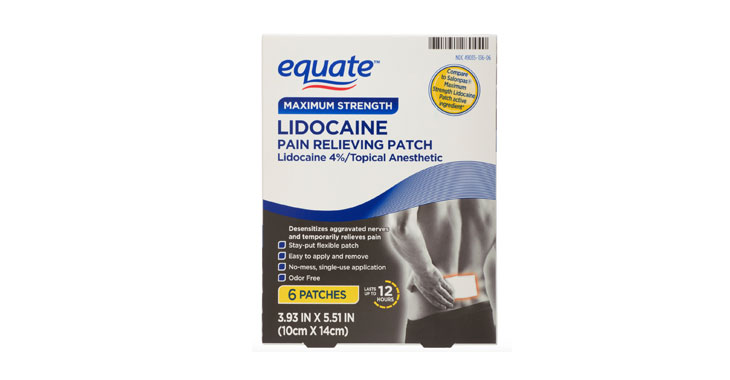
Allegations: Misleadingly marketing lidocaine pain relief patches
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: False “oil-free” claims
A closer look at the who, what, when, where and why.
Class-action lawsuits allege that the mAh ratings of several portable chargers are greatly exaggerated.
These claims are tough to swallow.
In honor of the Fourth of July, a reminder that not all “USA-made” products meet the legal definition.
While it’s the one touted on the box, stevia isn’t the only sweetener in this drink mix.