Walmart
TINA.org investigations into Walmart have revealed that the retail giant repeatedly engaged in false and deceptive Made in USA marketing on its website, and used undisclosed stealth marketing directed at…
December 2013: This case was voluntarily dismissed When a complaint is dismissed without prejudice, an amended version of the complaint can be refiled., the reasons for which have not been disclosed.
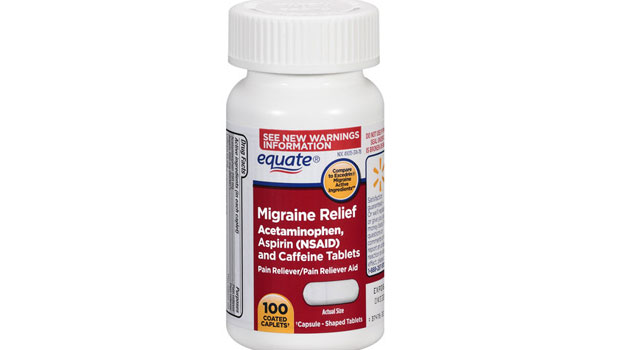
September 2013: A class-action lawsuit was filed against Walmart for allegedly deceptively marketing the over-the-counter pain reliever Equate Migraine Pain Reliever. Specifically, plaintiffs claim that Walmart promotes Equate Migraine as a stronger medicine than Equate Headache that will more effectively relieve migraines when, according to the complaint, both Equate Headache and Equate Migraine contain the same amounts of the same ingredients. (Cooper et al. v. Wal-Mart Stores, Inc., Case No. 13-cv-05446, D.N.J.).
For more of TINA.org’s coverage of Equate Migraine, click here.
TINA.org investigations into Walmart have revealed that the retail giant repeatedly engaged in false and deceptive Made in USA marketing on its website, and used undisclosed stealth marketing directed at…
Allegations: Falsely marketing sunscreens as “Reef Friendly”
Allegations: Falsely marketing sunscreens as “Reef Friendly”
Allegations: Misleadingly representing that products were safe when they contain, or were at risk of containing, the carcinogen benzene
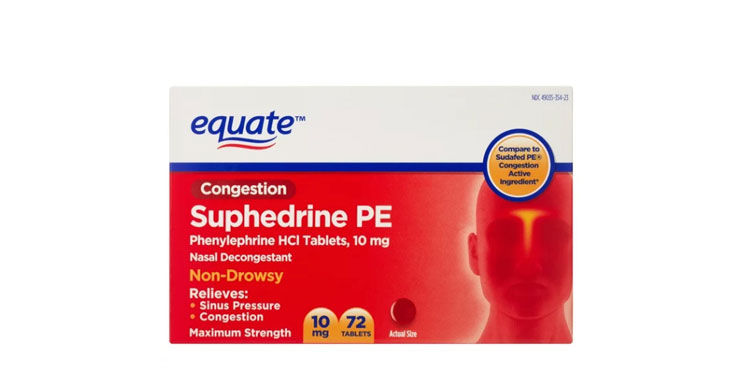
Allegations: Falsely marketing products as non-drowsy
Allegations: Falsely marketing that devices accurately measure blood pressure
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that medicines relieve nasal congestion and sinus pressure
Allegations: Falsely marketing that medicines combat congestion and other sinus issues
Allegations: Falsely marketing that medicines relieve nasal decongestion
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Misleadingly marketing medicines as “non-drowsy” when an ingredient in them causes drowsiness
Allegations: Falsely marketing sunscreens as “hypoallergenic” when they contain a significant amount of allergens, irritants, and other damage-causing chemicals
Allegations: Falsely marketing the product treats minor cuts and abrasions when scientific evidence shows it does not have such treatment capabilities
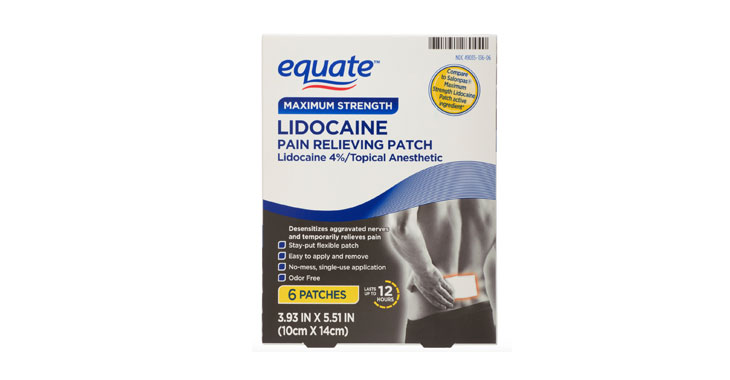
Allegations: Misleadingly marketing lidocaine pain relief patches
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: False “oil-free” claims
TINA.org has tracked more than 150 lawsuits alleging greenwashing.
An FDA panel’s recent findings has led to a flood of lawsuits.
Peter Adams, Marketing Dive
Universe of Play’s removal follows action led by TINA.org – and inaction from self-reg group.
Will it enforce them this time?