CVS
TINA.org investigated the marketing used to promote life’sDHA™, a supplement sold by CVS Pharmacy under the name Algal-900 DHA, and found that the marketing deceptively implied the product could improve memory…
Kleiman et al. v. CVS Health Corp.
23-cv-4727, D.S.C.
(Sept. 2023)
CVS cold and flu medicines
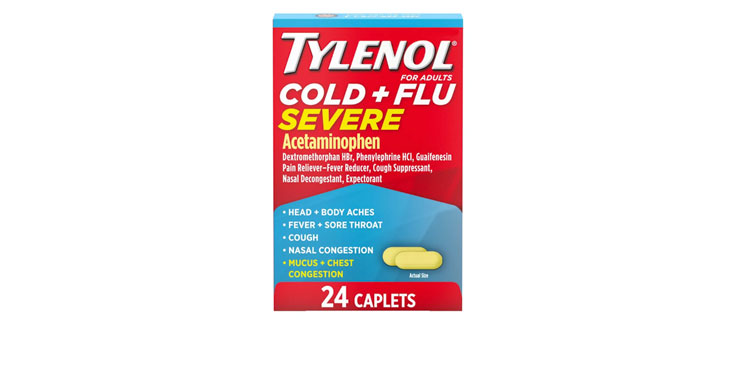
Falsely marketing that medicines treat congestion and other sinus issues when the active ingredient (phenylephrine) is not an effective decongestant
Pending
TINA.org investigated the marketing used to promote life’sDHA™, a supplement sold by CVS Pharmacy under the name Algal-900 DHA, and found that the marketing deceptively implied the product could improve memory…
Allegations: Falsely marketing that products contain “No Artificial Preservatives”
Allegations: Falsely marketing that products treat nail fungus
Allegations: Misleadingly marketing CVS Health Toddler Beginnings
Allegations: Falsely advertising the accuracy of ovulation test kits
Allegations: Falsely marketing products as “non habit-forming”
Allegations: Failing to disclose that products contain harmful synthetic chemicals known as PFAS
Allegations: Failing to disclose products contain the carcinogen benzene
Allegations: Falsely marketing products as covered in yogurt and healthy when the coating is actually “candy-like” and contains several unhealthy ingredients
Allegations: Misleadingly representing that products were safe and tested by dermatologists when they contain, or were at risk of containing, the carcinogen benzene
Allegations: Marketing products as sterile and safe when they have been contaminated with bacteria that may cause eye infections that can lead to partial vision loss or blindness
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing that medicines treat nasal congestion
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Defrauding consumers by asking them to make donations to the American Diabetes Association on checkout screens when all or part of the donated money went to CVS
Allegations: Falsely marketing that phenylephrine products treat congestion and other cold and flu symptoms
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing products as decongestants
Allegations: Falsely marketing that products treat symptoms of pink eye
Allegations: Falsely marketing that medicines treat nasal congestion
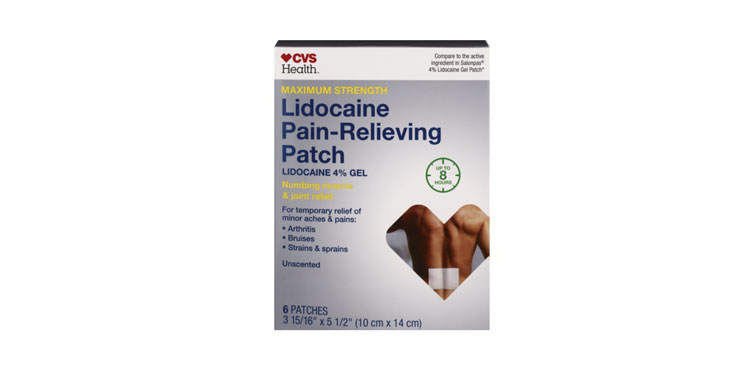
Allegations: Misleadingly marketing that patches provide “Maximum Strength” doses of lidocaine and up to 8 or 12 hours of pain relief
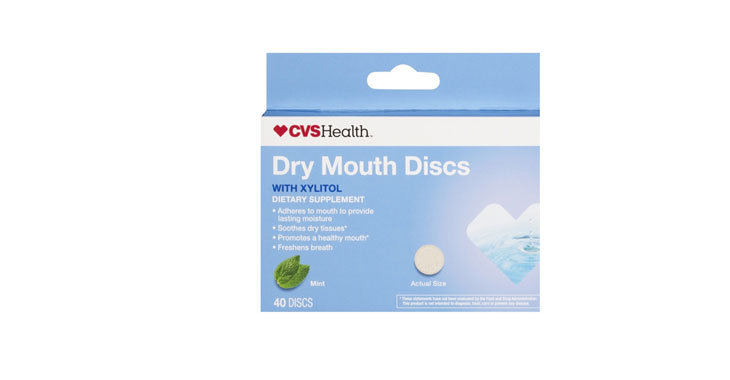
Allegations: Marketing that products promote a healthy mouth when using them contributes to various oral health issues
Allegations: Making misleading claims about the ingredients and capabilities of the toothpaste
Allegations: Falsely marketing products as if they repair gums and reverse gingivitis when the active ingredient does not provide such benefits
Allegations: Marketing products as safe pain relievers for pregnant women without warning consumers that scientific evidence shows prenatal exposure to APAP can cause neurodevelopmental disorders in children
Allegations: Falsely marketing hydrogen peroxide as a treatment for minor cuts and abrasions
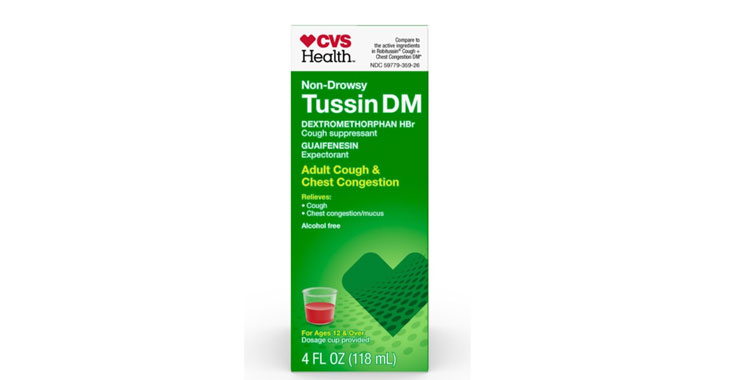
Allegations: Falsely marketing medicine as “non-drowsy” when an ingredient causes drowsiness
Allegations: Deceiving consumers about the proper use for its cotton swabs and failing to adequately disclose the risks associated with using cotton swabs to clean in the ear canal
Allegations: Failing to disclose that products may contain a dangerous substance that increases the risk of serious adverse health consequences and death
Allegations: Failing to disclose that products contain a harmful substance and may increase the risk of contracting invasive infections
Allegations: Falsely marketing that hand sanitizers “kill[] 99.99% of germs” when scientific evidence shows that alcohol-based hand sanitizers do not kill many types of germs
Allegations: Falsely marketing that products contain more protein than they actually do
Allegations: Failing to disclose products contain the carcinogenic chemical benzene
Allegations: Misleadingly marketing products as “maximum strength” when similar products from competitors contain more lidocaine
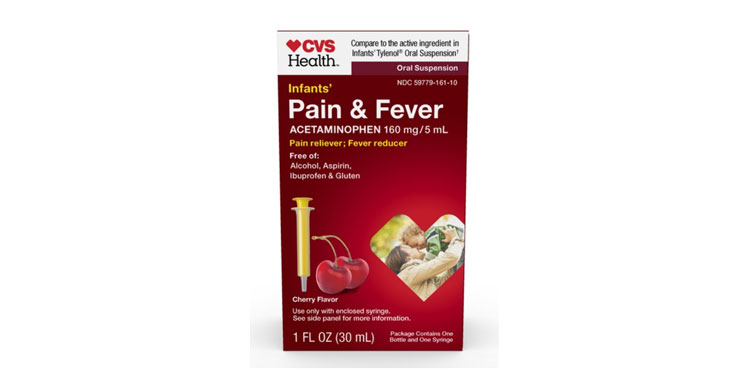
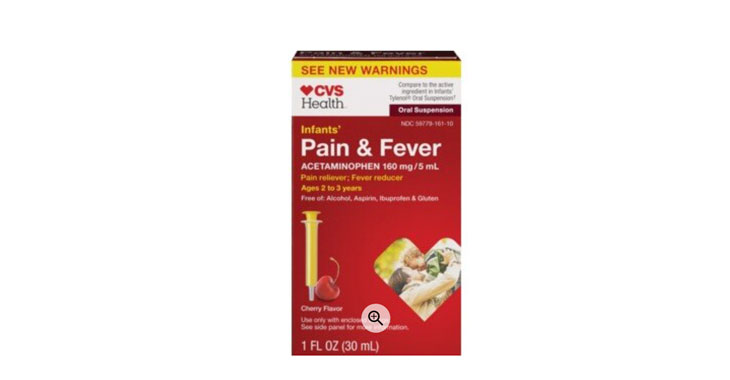
Allegations: Misleadingly marketing the infants’ product as specifically formulated for infants when it has the same concentration of acetaminophen as the children’s version
Allegations: Falsely marketing medicines as “non-drowsy” when the active ingredient in them causes drowsiness
Allegations: Misleadingly marketing that the active ingredients in sunscreens are zinc oxide minerals when there are also chemical active ingredients in the sunscreens
Allegations: Misleadingly marketing that the product is specifically formulated for infants and charging more for the infants’ product when it contains the same formulation of the same active ingredient in…
Allegations: Misleadingly marketing that hand sanitizers “Kill[] 99.99% of Germs” when there is no scientific evidence to support such claims
These definitions are a joke.
An FDA panel’s recent findings has led to a flood of lawsuits.
This ad hits different when you read the fine print.
FDA targets companies selling eye drops illegally marketed to treat conditions like pink eye.
Plaintiffs allege packaging misrepresents lidocaine dosages as ‘maximum strength,’ among other things.