ChapStick Natural Claims
Allegations: False natural claims
In May 2020, a class-action lawsuit was filed against GlaxoSmithKline for allegedly falsely advertising the medication Boostrix in what has been referred to as “The Big Bad Cough” advertising campaign. Plaintiffs claim that the company falsely advertises that Boostrix makes users less likely to become infected with and transmit whooping cough when, according to the complaint, the medicine generates antibodies to only five of the thousands of the antigens of whooping cough, which means people who take Boostrix may still become infected and transmit whooping cough even if they are showing few or no symptoms. (DeCostanzo et al v. GlaxoSmithKline PLC, Case No. 20-cv-2284, E.D.N.Y.)
For more of TINA.org’s coverage of medicine, click here.
Allegations: False natural claims
Allegations: Falsely marketing products as containing “Natural Flavors”
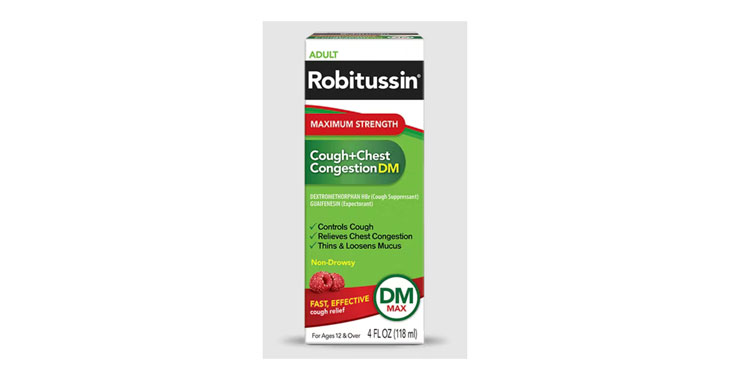
Allegations: Falsely marketing medicine as “Non-Drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing that products are made with natural flavors
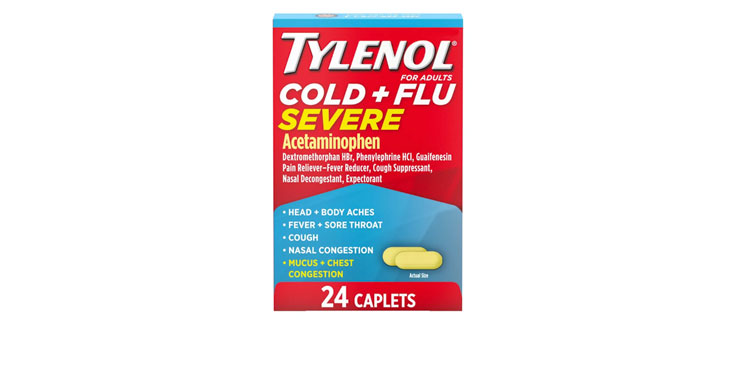
Allegations: Falsely marketing that medicines treat nasal congestion
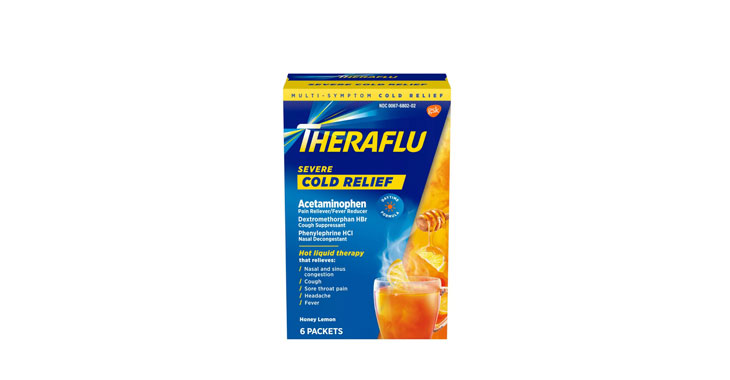
Allegations: Misleadingly marketing the ingredients in and the health benefits provided by products
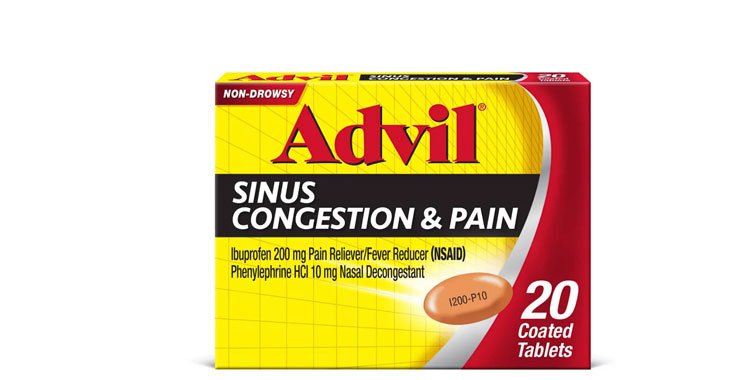
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing that medicines treat congestion
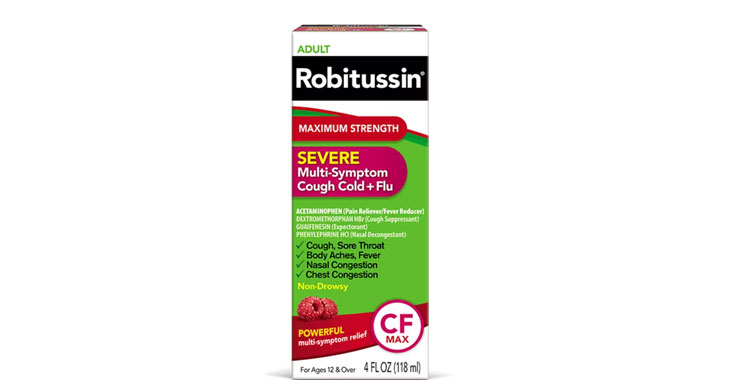
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing medicines as decongestants
Allegations: Falsely marketing products as decongestants
Allegations: False natural claims
Allegations: Falsely marketing products as non-drowsy when an ingredient in them causes drowsiness
Allegations: Misleadingly marketing Abreva cold sore treatments
Several products marketed as ‘non-drowsy’ contain an ingredient that causes drowsiness, lawsuits claim.
Lip balm’s own directions seem to contradict its “8 Hour Moisture” labeling claim.
Cue the play-off music.
Is Big Pharma marketing a drug to help aging men with low sex drives or really selling a made-up disease?
GSK will pay out $105 million in off-label use case.