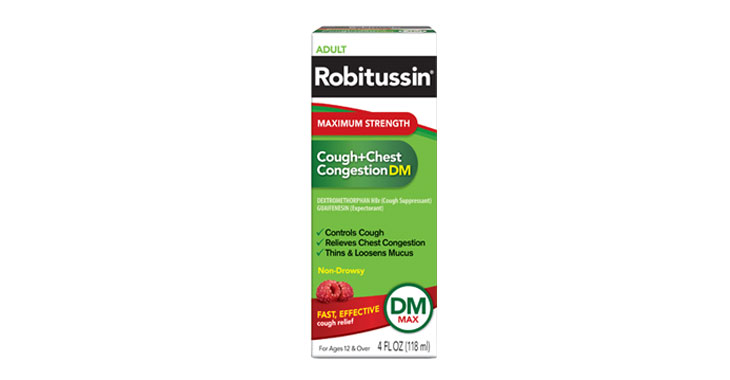
CATrends: OTC Cold and Flu Medicines Falsely Marketed as ‘Non-Drowsy’
Several products marketed as ‘non-drowsy’ contain an ingredient that causes drowsiness, lawsuits claim.
Several products marketed as ‘non-drowsy’ contain an ingredient that causes drowsiness, lawsuits claim.
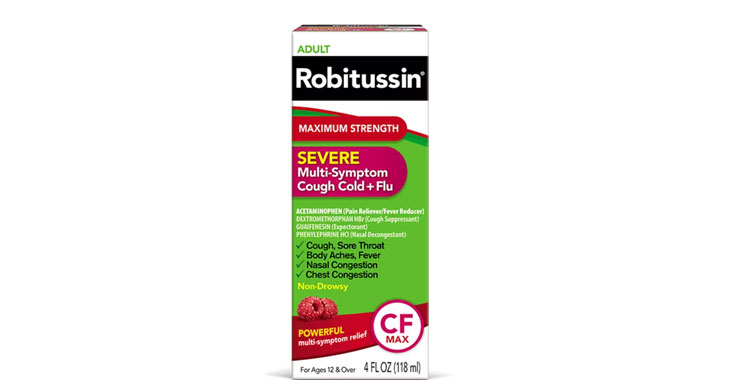
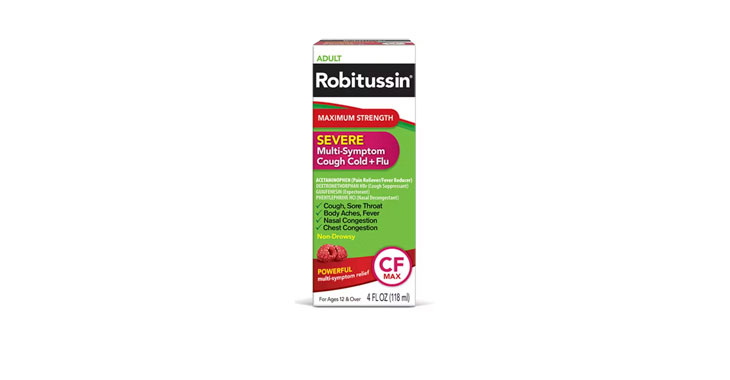
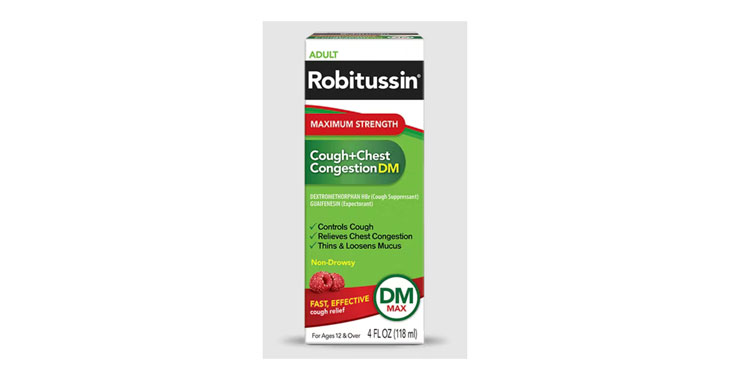
Allegations: Falsely marketing medicine as “Non-Drowsy” when the active ingredient causes drowsiness
Allegations: Misleadingly marketing the ingredients in and the health benefits provided by products
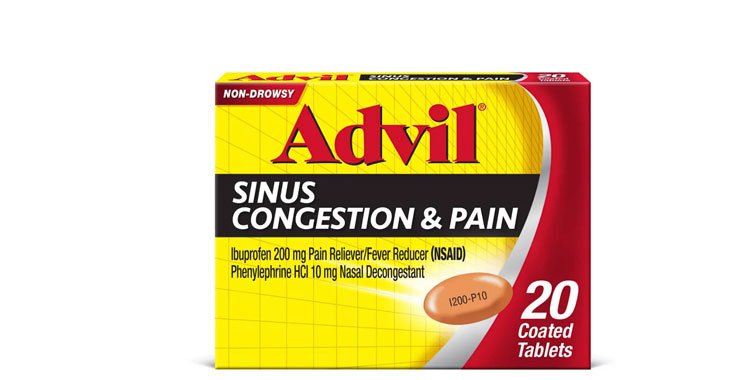
Allegations: Falsely marketing that medicines treat congestion
Allegations: Falsely marketing that medicines relieve nasal congestion and sinus pressure
Allegations: Falsely marketing medicines as nasal decongestants
Allegations: Falsely marketing that medicines relieve nasal congestion
Allegations: Falsely marketing products as non-drowsy when an ingredient in them causes drowsiness
Allegations: Deceptively marketing products as “Maximum Strength” when the dosage of the active ingredients is less than or the same as the regular strength products