LifeWave
TINA.org investigated LifeWave as part of its larger investigations into unsubstantiated disease-treatment claims and atypical income claims used by DSA-member companies.
To read more about TINA.org’s DSA Health Claims Investigation, click here.
To read more about TINA.org’s DSA Income Claims Investigation, click here.
How We Took Action
2025
March 3
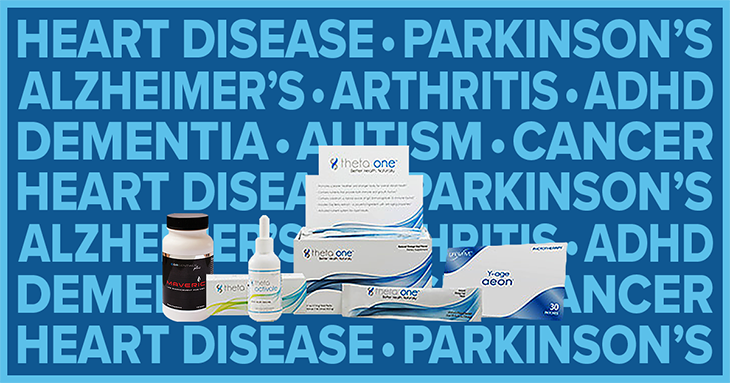
The DSSRC issues a case decision finding that LifeWave uses atypical earnings claims to market its business opportunity and unsubstantiated health claims to market products.
2021
October 26
The FTC sends LifeWave a Notice of Penalty Offenses Concerning Money-Making Opportunities putting the company on formal notice that it is an unfair or deceptive trade practice to misrepresent that profits or earnings are the ordinary, typical or average profits or earnings made by participants, and to fail to disclose conditions affecting income, such as expenses borne by the participants, among other things.
June 30
TINA.org sends a letter, along with a list of more than 660 direct selling companies that includes LifeWave, to the FTC urging it to implement a penalty offense program targeting the direct selling industry and its market-wide practice of using deceptive earnings representations and false health claims.
2017
December 18
As part of its 2017 investigation into all DSA-member companies, TINA.org sends a letter to LifeWave regarding its use of false and unsubstantiated income claims to promote its business opportunity.
2016
December 1
LifeWave responds to TINA.org.
November 22
As part of its 2016 investigation into all DSA-member companies selling nutritional supplements, TINA.org sends a letter to LifeWave regarding its use of inappropriate health claims to market products.