SeraRelief CBD products
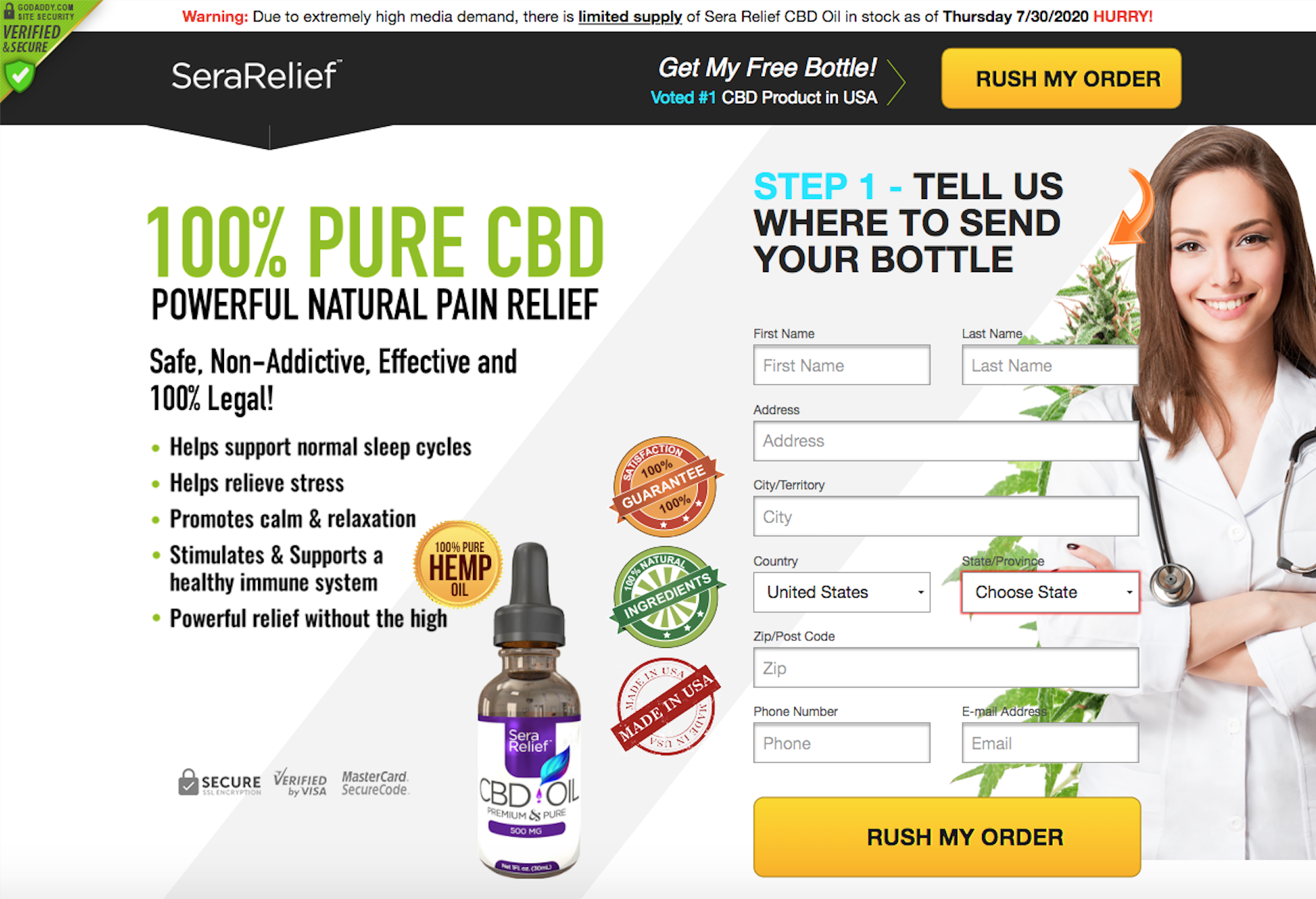
Be wary of “free trials” for CBD gummies and oils advertised on the internet.
FDA reminds marijuana marketers that outing unproven treatment claims remains high on its list.
As more states move to legalize medical marijuana — at 29, there are now more states that have than haven’t — some companies are taking the proliferation of state-sanctioned pot for medicinal purposes as a cue to market their own marijuana-based products to treat or cure a number of serious diseases, including cancer.
These companies include Natural Alchemist, which makes online claims that the cannabidiol or CBD in its drops and capsules “combats tumor and cancer cells,” in addition to providing therapeutic benefits in the treatment of Alzheimer’s, Parkinson’s, PTSD, anxiety, diabetes, and schizophrenia among other conditions. Unlike tetrahydrocannabinol or THC, CBD is the compound in marijuana that does not make people high.
Yet the FDA has not approved any product containing or derived from botanical marijuana for the treatment of any health condition. That puts the company’s health claims square in the category of Only FDA-approved drugs can be marketed as having the ability to diagnose, cure, treat, prevent or mitigate a disease., as the FDA recently informed the California-based firm in a warning letter dated Oct. 31.
“Substances that contain components of marijuana will be treated like any other products that make unproven claims to shrink cancer tumors,” said FDA Commissioner Scott Gottlieb in a statement. “We don’t let companies market products that deliberately prey on sick people with baseless claims that their substance can shrink or cure cancer and we’re not going to look the other way on enforcing these principles when it comes to marijuana-containing products.”
At the same time, the FDA is cognizant of early research on the potential promise medical marijuana holds out for sufferers of serious conditions such as epilepsy, anxiety and schizophrenia. And while the agency has yet to green-light a plant-based marijuana product, it has approved medications containing synthetic THC for the treatment of anorexia associated with weight loss in AIDS patients and for chemotherapy-related nausea and vomiting.
“We recognize that there’s interest in developing therapies from marijuana and its components, but the safest way for this to occur is through the drug approval process — not through unsubstantiated claims made on a website,” Gottlieb said.
Natural Alchemist, which has yet to wipe all of the problematic health claims cited in the FDA’s warning letter, wasn’t the only online purveyor of miracle marijuana-based products that received mail from the agency. Read about the others here: Charlotte’s Web, Green Roads Health, That’s Natural.
Find more of our coverage on marijuana here.
Our Ad Alerts are not just about false and deceptive marketing issues, but may also be about ads that, although not necessarily deceptive, should be viewed with caution. Ad Alerts can also be about single issues and may not include a comprehensive list of all marketing issues relating to the brand discussed.
Be wary of “free trials” for CBD gummies and oils advertised on the internet.
CBD “super store” brochure is chock full of unapproved disease-treatment claims.
Disease-treatment claims vanish in wake of FTC warning letter.