
James ‘Jay’ Noland’s Latest Ventures Raise Familiar Concerns
Permanently banned from MLM, Noland has found other ways to exploit consumers.
 Discovery
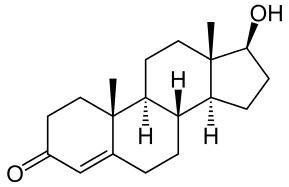
DiscoveryIsolated testosterone is synthesized and introduced to treat hypogonadism.
 Hypogonadism Therapy
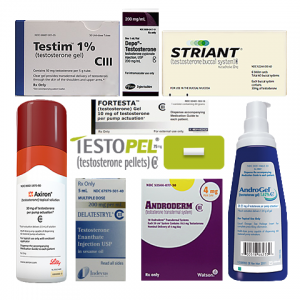
Hypogonadism TherapyFDA approves Delatestryl to treat conditions associated with a deficiency or absence of testosterone, primarily known as hypogonadism. It is the only condition for which testosterone replacement therapy (TRT) is approved by the FDA. By 2010, the agency has approved at least eight more drugs — Testopel, Depo-Testosterone, Androderm, AndroGel, Testim, Striant, Axiron and Fortesta – to treat the uncommon condition.
 New Disease Penned
New Disease PennedBig Pharma, in an attempt to persuade men and their doctors that there is a new condition termed “Low T” or “Andropause,” which medical critics say is simply the aging process, engages in direct-to-consumer advertising and physician education programs to expand the market for the drugs.
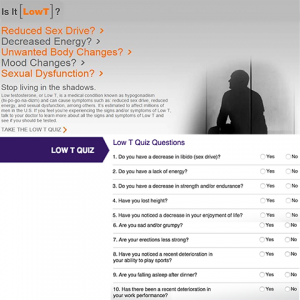 Self Diagnosis
Self DiagnosisA geriatrics doctor at the St. Louis University School of Medicine develops a quiz in exchange for a $40,000 grant designed to help men self-diagnose whether they need testosterone. The quiz is featured on AndroGel’s website and TRT sites.

The FDA warns AbbVie for the second time that claims for AndroGel suggesting that the product is indicated for men with “age-associated hypogonadism” or “Andropause” are misleading.
 Blowing the Whistle
Blowing the WhistleFederal whistleblower suit against AndroGel alleges that the company made millions by marketing testosterone drugs in the U.S. for conditions not approved by the FDA, offered kickbacks to physicians to prescribe the drug, and trained them to misstate diagnoses so that Medicaid would pay for the medications.
 Millions in Sales
Millions in SalesAndroGel sales reach $300 million.
 Dosing Up
Dosing UpMore than 100 million doses of testosterone are prescribed.
 Unapproved Uses
Unapproved UsesFDA warns Auxilium’s predecessor, Slate, that its “Reclaim Your Life” marketing campaign for Testopel is misleading because it promotes “unapproved uses of Testopel.”
 Risky Business
Risky BusinessNearly one in 25 men in their 60s are taking testosterone.
 Kansas resident Robert Cripe suffers a spinal stroke and is paralyzed. He sues Androgel, alleging it’s the cause. He later becomes one of thousands of men suing testosterone manufacturers.
Kansas resident Robert Cripe suffers a spinal stroke and is paralyzed. He sues Androgel, alleging it’s the cause. He later becomes one of thousands of men suing testosterone manufacturers.
 Advertising Blitz
Advertising BlitzDrug makers spend more than $100 million to advertise testosterone products and sales reach $2 billion. The Colbert Report takes industry to task.
 A New Day
A New DayAxiron commercial by Grey New York entitled “A New Day” wins a bronze medal at the DTC National Advertising Awards.
 ‘Uncontrolled Experiment’
‘Uncontrolled Experiment’The Journal of the American Medical Association publishes scathing article on testosterone prescriptions calling Low T “[a] mass, uncontrolled experiment, which invites men to expose themselves to the harms of a treatment unlikely to fix problems which they may not really have.”
 Alarming Health Risks
Alarming Health RisksSales of TRT drugs top $2 billion, and there are more than 6.5 million prescriptions written.
 Lawsuits against drug companies start to mount. In one, Ohio-based health insurer Medical Mutual asserts that the major TRT manufactures colluded in a “decade-long deceptive marketing scheme to transform the male aging process into a curable disease” and concealed side effects.
Lawsuits against drug companies start to mount. In one, Ohio-based health insurer Medical Mutual asserts that the major TRT manufactures colluded in a “decade-long deceptive marketing scheme to transform the male aging process into a curable disease” and concealed side effects.
 Feds Issue Warning
Feds Issue WarningThe FDA announces it is requiring manufacturers of testosterone to include a warning about health risks, including pulmonary embolism.
 Disease Mongering Persists
Disease Mongering PersistsThe Journal of the American Geriatrics Society lambasts the mass marketing and permissive prescribing of testosterone as “disease mongering.”
 FDA Safety Alert
FDA Safety AlertFDA issues a safety announcement, specifically noting that “prescription testosterone products are approved only for men who have low testosterone levels caused by certain medical conditions” and calls on drug makers to conduct clinical trials of testosterone products marketed to older men.
The American Medical Association votes in favor of a ban on direct-to-consumer drug advertising. Said AMA Board Chair Patrice A. Harris: “Today’s vote in support of an advertising ban reflects concerns among physicians about the negative impact of commercially-driven promotions….”
 Lawsuits Mount
Lawsuits MountThe first bellwether trials of the more than 5,000 lawsuits filed against manufacturers will begin in the fall with AbbVie as the defendant. Big Pharma, is keeping close watch on cases and a possible First Amendment defense – as used in Amarin Pharma’s complaint against the FDA.
Permanently banned from MLM, Noland has found other ways to exploit consumers.
The best wake up songs. The best cups of coffee. The best part of wakin’ up.
Pulling back the curtain on this official-sounding website.

