Alkaline Water Plus
Are there really any benefits to drinking alkaline water?
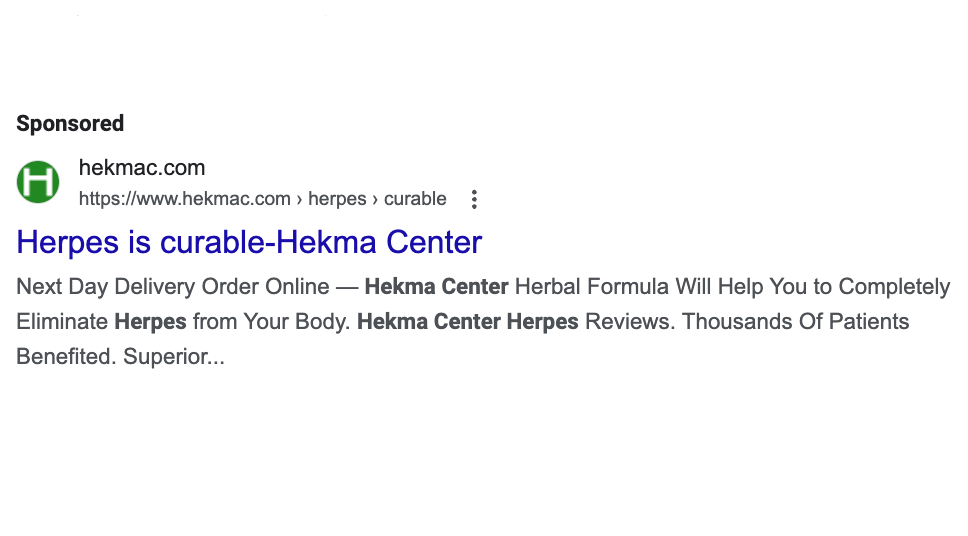
Herpes is a viral infection that is estimated to affect over half of the American population and for which there is no known cure. But that’s not what Hekma Center, a Delaware-based supplement company, is saying.
In this YouTube ad, for example, the company claims that its herbal formula will “help you to completely eliminate the herpes virus from your body (Genital Herpes and Oral Herpes).” And its Google ads assert that, with Hekma, “Herpes is curable” (a message that is in stark contrast to the medical community’s position).
So does this $1,500 herpes formula actually work?
Although Hekma Center claims to have a “scientific research team” creating its products, TINA.org found no studies cited on its website at all.
And while Hekma Center brags about an abundance of certifications, there is a notable one missing – an FDA approval.
Without this, Hekma Center cannot legally claim to cure, mitigate, treat or prevent herpes or any disease for that matter, a fact that was clearly articulated in a 2023 FDA warning letter concerning the company’s promotion of products to treat anemia, diabetes, high blood pressure and more.
It’s also worth noting that despite the FDA warning, the company has continued to make health claims for a variety of other products. In addition to the herpes formula, the company’s Instagram page promotes reviews that claim the company’s products can treat anything from ADHD to liver problems.
And in case you’re wondering, yes, the company is responsible for claims made in consumer reviews if those reviews are then used in marketing, like here. According to the FTC:
[A]dvertisers should not make claims through consumer testimonials … that would be deceptive or couldn’t be substantiated if the advertiser made them directly. It’s not enough that a testimonial represents the honest opinion or experience of an endorser. Under FTC law, advertisers also must have appropriate scientific evidence to back up the underlying implied claim that the product is effective and will work for buyers as it did for the endorser.
Hekma Center did not respond to TINA.org’s request for comment.
The bottom line
Remember, it is illegal for companies to claim a supplement can treat any disease, or to make any health claim without the proper scientific evidence. Consumers should always consult with their health care provider if they’re seeking treatment for a health condition.
Find more of our coverage on supplements here.
Our Ad Alerts are not just about false and deceptive marketing issues, but may also be about ads that, although not necessarily deceptive, should be viewed with caution. Ad Alerts can also be about single issues and may not include a comprehensive list of all marketing issues relating to the brand discussed.
Are there really any benefits to drinking alkaline water?
What you need to know about this purported hangover remedy.
Spam email leads to a fake endorsement from Dr. Oz, among other celebrities.