Best Reader Tips of 2021
This year reader tips led to dozens of ad alerts, as well as a complaint to regulators.
FDA drafts recommendations on how the industry should use social media to market products.
The FDA has its concerns about Twitter; namely, how big pharma and others use the character-restricted platform to market its products. At the center of the issue is the challenge to communicate both the benefits and risks of a product – and other consumer information the FDA deems important – in a space limited to 140 characters. Thomas Abrams, director of the FDA’s Office of Prescription Drug Promotion, writes:
In today’s world, in addition to traditional sources of medical product information, patients and health care providers regularly get information about FDA-regulated medical products through social media and other Internet sources… But, no matter the Internet source used, benefit claims in product promotions should be balanced with risk information.
To that point, the FDA has released a draft guidance on what the industry should be doing when it tweets, uses paid search engine results, or otherwise advertises prescription drugs or medical devices on the web where space is limited. The guidelines aren’t meant for product websites, individual product pages on social media, and online web banners where space isn’t limited. The document, which is in a feedback stage until Sept. 16, would not necessarily carry any legal implications, but would rather serve as something that is suggested or recommended, though not required.
As an example of what the FDA would consider acceptable on Twitter, the draft guidance offers up a sample tweet for a fictitious drug called NoFocus:
NoFocus (rememberine HCl) for mild to moderate memory loss-May cause seizures in patients with a seizure disorder www.nofocus.com/risk
The tweet works, according to the FDA, because it presents the most serious precaution associated with the drug alongside the prescribed treatment. It also contains a link to a full description of potential risks, and it includes the brand and generic name. Also, notice the dash between “loss” and “May.” The draft ordinance allows for the use of punctuation marks, common abbreviations and other symbols (like an ampersand in place of “and”) to help address character space constraints.
Of course, for drugs whose label details several potentially serious health risks – take Viagra, for example – the challenge to cram it all in a 140-word tweet becomes a difficult if not impossible task. The draft guidance suggests not to fight that fight:
If a firm concludes that adequate benefit and risk information, as well as other required information, cannot all be communicated within the same character-space-limited communication, then the firm should reconsider using that platform for the intended promotional message.
TINA.org investigated further and searched for some live tweets that would potentially run afoul of the draft guidance. We found the following post on the Twitter page for Pfizer: 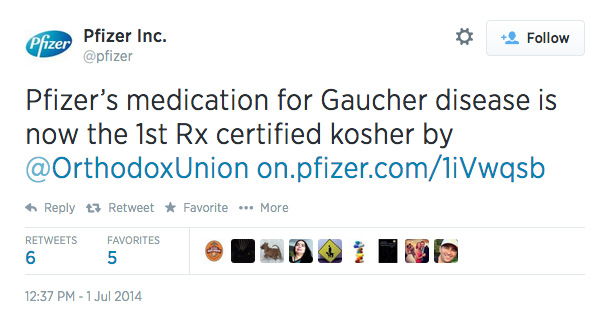 It would seem that this tweet for an FDA-approved drug called Elelyso immediately comes up short of the draft guidance’s recommendations because it fails to disclose any potential health risks, and thus should not be posted to Twitter.
It would seem that this tweet for an FDA-approved drug called Elelyso immediately comes up short of the draft guidance’s recommendations because it fails to disclose any potential health risks, and thus should not be posted to Twitter.
If you come across a tweet or paid search engine result (such as you’d find on Google or Yahoo) that you think may violate the FDA’s draft ordinance, tweet at TINA.org @TruthinAd, leave a message on our Facebook page, or alert us here.
This year reader tips led to dozens of ad alerts, as well as a complaint to regulators.
Supplement MLM takes down dozens of deceptive claims following TINA.org investigation.