Gladiators star under fire for ‘promoting brand accused of deceptively marketing body-building food supplements to kids’
Madison Burgess, Daily Mail
Ad Watchdog Finds Widespread Deceptive Marketing.
An investigation into the menopause supplement industry by consumer advocacy organization truthinadvertising.org (TINA.org) has revealed a hotbed of deceptive advertising. The ad watchdog has amassed nearly 2,000 examples of problematic health claims promoting Pharmavite’s Equelle and Alliance Pharmaceuticals’ Amberen, filing complaints with both the FTC and the FDA. These findings follow previous TINA.org investigations into brands, including Gwyneth Paltrow’s Goop and California-based MLM Modere, that TINA.org found deceptively promoted menopause supplements.
With approximately 6,000 women in the U.S. reaching menopause every day, there is a lucrative market for products advertising relief from symptoms like hot flashes, night sweats and anxiety. In fact, the global menopause supplement industry is expected to surpass $22 billion by 2028.
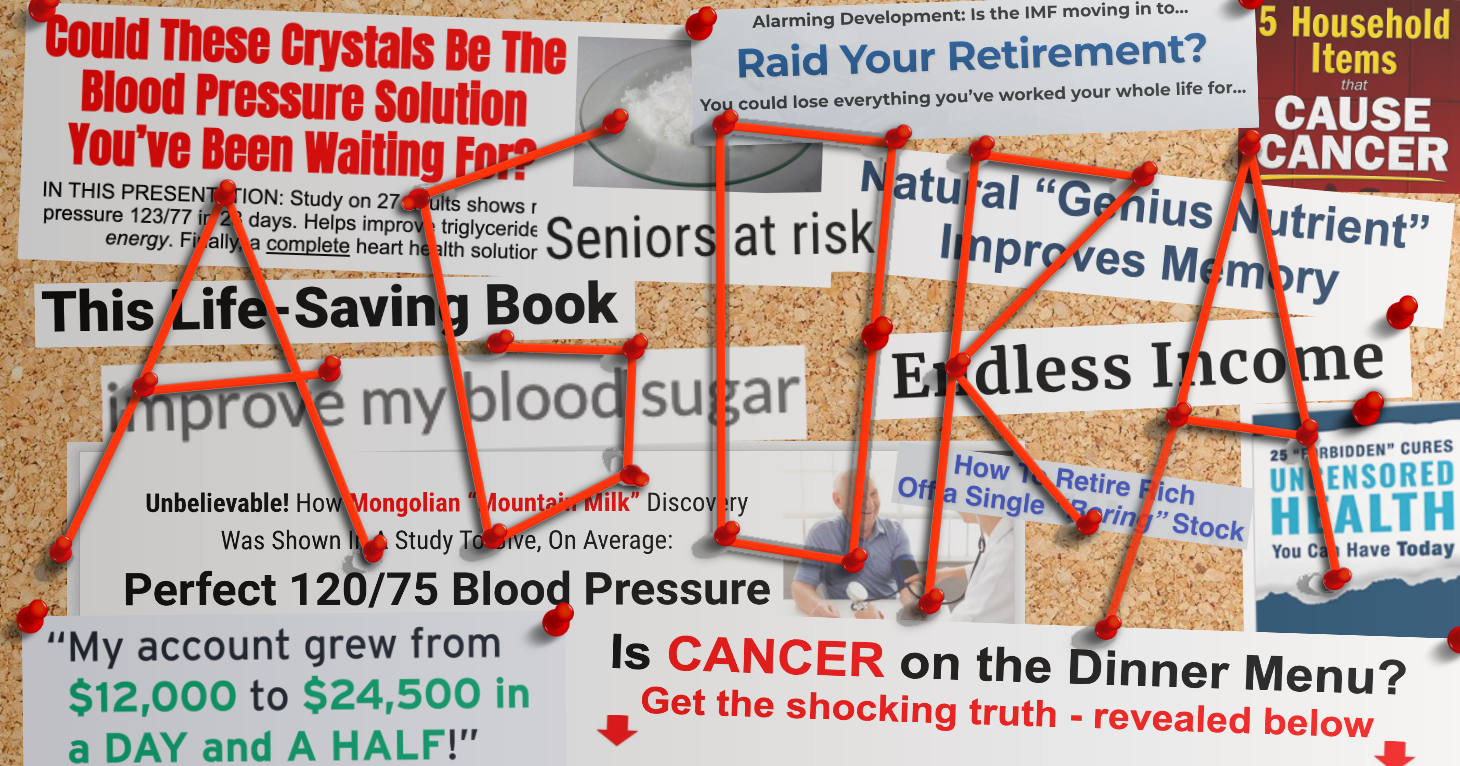
The problem? Supplements have not been found to be effective at treating the symptoms of menopause. What’s more, many supplement companies are making menopause relief claims in marketing materials without proper scientific support in violation of FTC law. And because some of the claims rise to the level of drug claims, the marketers are also running afoul of FDA regulations.
“These supplement brands are exploiting those suffering with the symptoms of menopause by deceptively promoting products that are expensive but have not reliably been shown to work,” said TINA.org Executive Director Bonnie Patten. “It’s time to put a stop to this epidemic of predatory marketing.”
Through its website, social media channels, national TV and Google ads, Equelle “guarantees” menopause relief and boasts that it is “clinically shown” to treat numerous symptoms such as severe hot flashes and excessive night sweats. Likewise, Amberen is promoted to relieve a host of symptoms from hot flashes and night sweats to anxiety, depression and low libido.
But TINA.org found that neither company could substantiate these claims with competent and reliable scientific evidence as required by law. Amberen’s violations could lead to the company paying millions in civil penalties as the company is already the subject of a previous court order regarding misleading menopause relief claims.
In addition to filing regulatory complaints against Equelle and Amberen, TINA.org sent letters to 100 other menopause supplement companies, notifying them of this deceptive marketing trend in their industry and urging them to review their marketing to ensure it complies with the law. The ad watchdog also issued a consumer alert with information about what to watch out for regarding health claims made by menopause supplement marketers.
To read more about deceptive marketing in the menopause supplement industry, see: https://truthinadvertising.org/articles/the-menopause-deception-epidemic/
If you are a member of the media looking to contact us, please email us at: press@tina.org
Madison Burgess, Daily Mail
MADISON, CONN. June 21, 2021 – Despite a $2 million settlement with the Federal Trade Commission in February 2021, publishing giant Monument & Cathedral Holdings, better known as Agora, and…
MADISON, CONN. April 28, 2020 – Truthinadvertising.org (TINA.org) has filed a complaint against multilevel marketing company Herbalife with the Federal Trade Commission (FTC) for deceptive claims that Herbalife products can…