Cartice
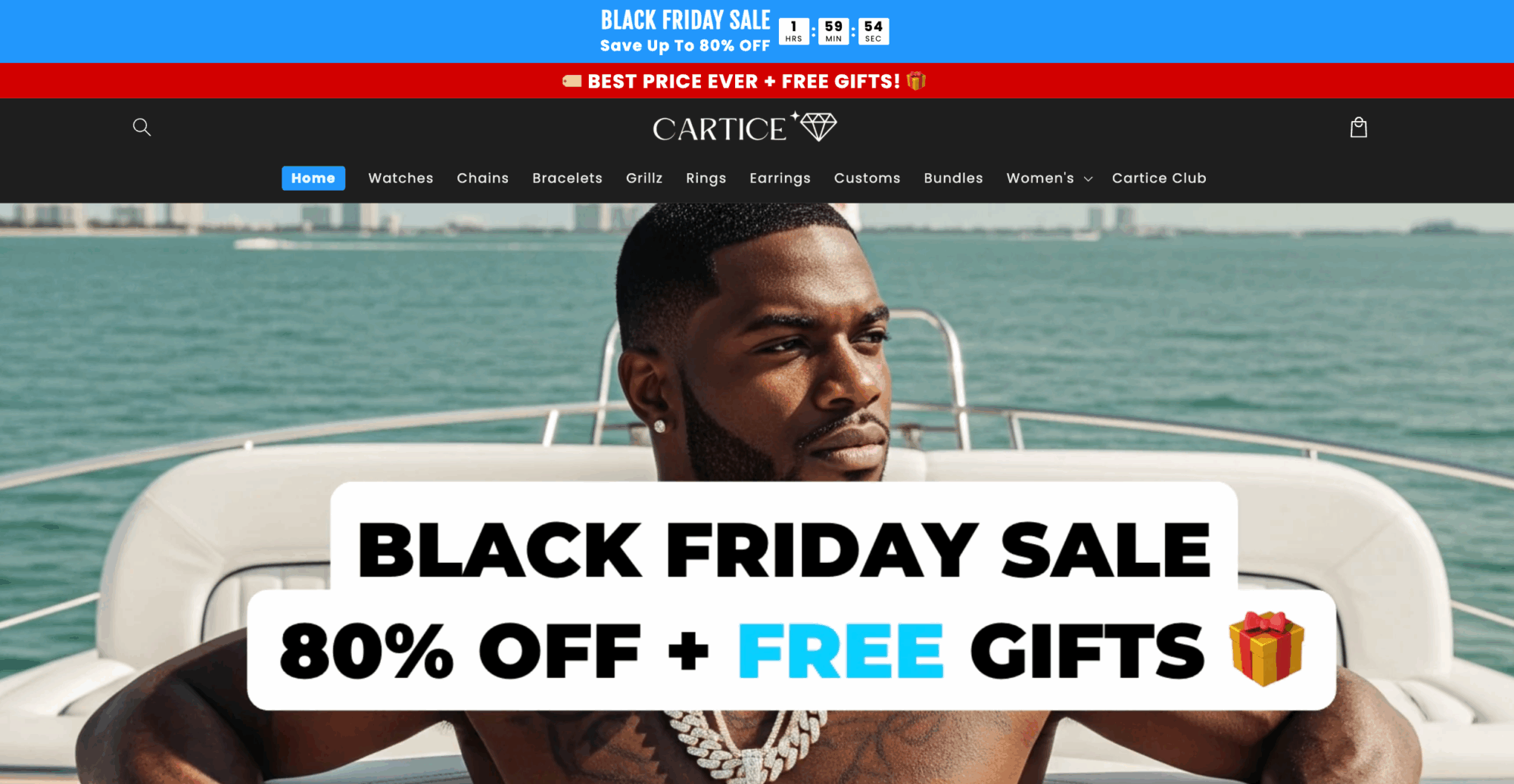
Watch out for this company’s sketchy sale.
January 2018: This action was voluntarily dismissed When a complaint is dismissed with prejudice, it cannot be refiled., the reasons for which have not been disclosed.
August 2017: The parties informed the Court that the appeal in the Jones case was voluntarily dismissed.
May 2015: The case was stayed because the resolution of an appeal pending in the Ninth Circuit, Jones v. ConAgra Foods, may impact class certification issues in this case.
February 2015: Plaintiffs filed an amended complaint making similar allegations (i.e., that the company allegedly fails to adequately disclose important information about TNS® products, including health and safety concerns associated with the ingredients in the products and the composition of the products).
June 2014: SkinMedica, Inc. moved to dismiss a class-action lawsuit against it. The complaint, which was originally filed in March 2014, alleges, among other things, that the company markets various skin rejuvenation products containing a human growth factor mix called NouriCel-MD® — including TNS Ultimate Daily Moisturizer, TNS Body Lotion, TNS Eye Repair, and TNS Lip Plump System – without adequately disclosing the health and safety concerns associated with the human growth factors contained in them. (Ruhnke et al v. SkinMedica, Inc. and Allergan, Inc., Case No. 14-cv-00420, C. D. CA.).
For more information about other class-action lawsuits regarding the advertising of beauty products and TINA.org’s coverage of the issue, click here.
Watch out for this company’s sketchy sale.
Some class-action settlements that left consumers out in the cold.
Don’t let this post-trip extension take you for a bumpy ride.
Free offer requires spending money.
The holiday shopping season is here. Tread carefully.