Home Depot’s Rental Damage Protection Fee
TINA.org staffer gets surprise charge.
November 2014: A federal judge granted final approval of this settlement.
February 2014: A federal judge preliminarily approved a settlement to a class-action lawsuit filed against several companies regarding the marketing of Sinus Buster products. The complaint, which was originally filed in 2012, alleges that, among other things, the companies misleadingly market the products as homeopathic, effective, clinically proven to be effective, and FDA approved when, in reality, none of these things are true.
According to the settlement terms, class members with proof of purchase may receive a full refund and class members without proof of purchase may receive $5 for up to 2 Sinus Buster products. In addition, the company agreed to injunctive relief, including adding specific language and disclaimers to the packaging and referring consumers to the company’s website for more information about dilution levels. (In Re: Sinus Buster Products Consumer Litigation, Case No. 12-cv-02429, E. D. NY.).
For more information about other class-action lawsuits regarding homeopathic remedies and TINA.org’s coverage of the issue, click here.
TINA.org staffer gets surprise charge.
TINA.org files complaint with NYC over company’s “$19.95” truck rentals.
Lawsuit cries fowl over preservative-free claims.
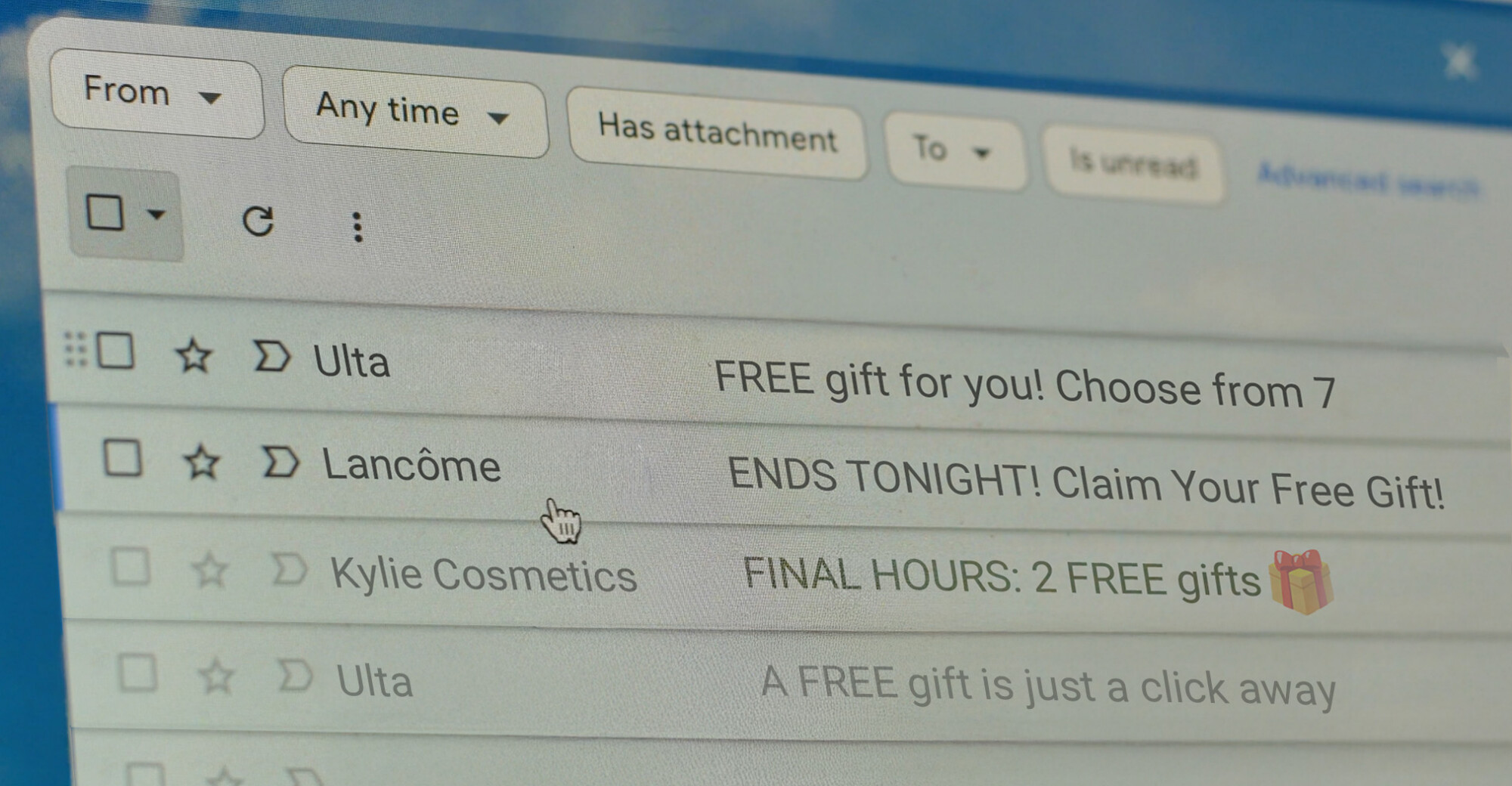
Lawsuits target misleading subject lines.
MADISON, CONN. Jan. 27, 2026 – Beverage giant Keurig Dr Pepper is deceptively marketing its single-serve K-Cup pods as “recyclable” in violation of state and federal laws, according to an…