www.boironusa.com
Allegations: Falsely representing that the website protects consumers’ privacy when it allows a third party to spy on visitors to its website for advertising purposes
January 2017: After a trial, judgment was entered in favor of the company because the plaintiff failed to prove that the company’s representations were false, misleading, or likely to deceive reasonable consumers. To learn more about the court’s decision, click here and here.
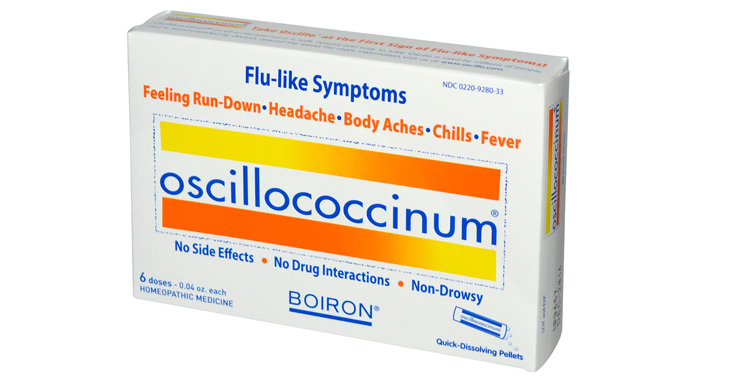
February 2014: A federal judge refused to certify the class in a class-action lawsuit filed against Boiron in 2011. The complaint claims that the company misleadingly represents that its Oscillococcinum and Children’s Oscillococcinum will relieve flu-like symptoms – including headaches, body aches, chills, and fevers – without scientific evidence to support such claims. The judge found that the named plaintiff, who claimed to have read the product label before purchasing it in the complaint and later testified that he read the label afterwards, could not adequately represent the class because his inconsistent statements put his credibility in issue and could harm the class. (Jovel et al v. Boiron, Inc.; Boiron USA, Inc.; and Labratories Boiron, Case No. 11-cv-10803, C. D. CA.).
For more information about other class-action lawsuits filed against Boiron and TINA.org’s coverage of the company, click here.
Allegations: Falsely representing that the website protects consumers’ privacy when it allows a third party to spy on visitors to its website for advertising purposes
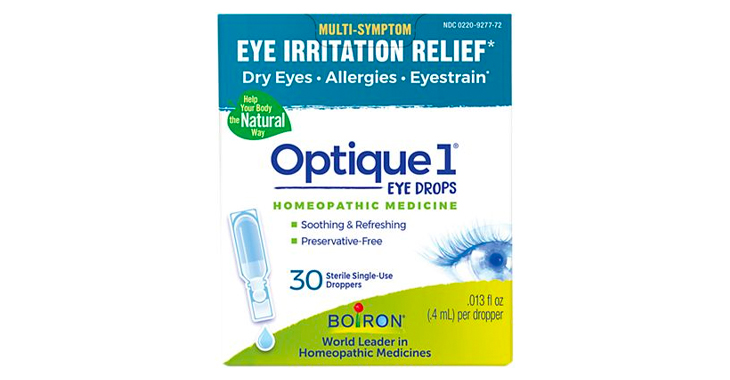
Allegations: Falsely marketing that products are “Homeopathic Medicine” that provide relief from eye irritation, dry eyes, allergies, and eye strain when they do not provide the advertised relief
TINA.org digs into shop’s purported Newport roots, and more.
TINA.org staffer gets surprise charge.
TINA.org files complaint with NYC over company’s “$19.95” truck rentals.
Lawsuit cries fowl over preservative-free claims.
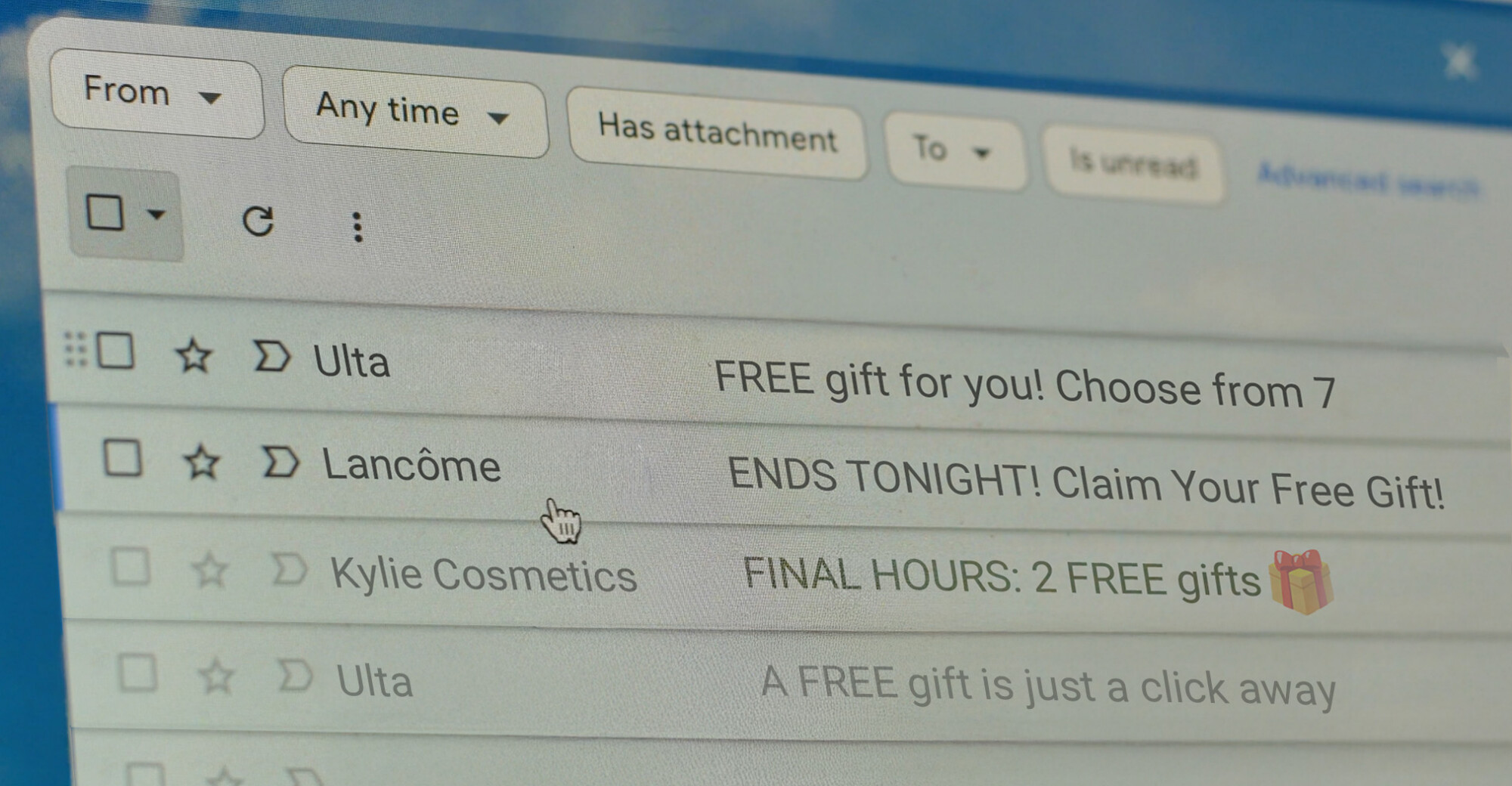
Lawsuits target misleading subject lines.