
Bluebonnet Chelated Magnesium
June 2014: The named plaintiff voluntarily dismissed this action When a complaint is dismissed without prejudice, an amended version of the complaint can be refiled.. The reasons for the dismissal have not been disclosed.
April 2014: A class-action lawsuit was filed against Bluebonnet Nutrition for allegedly deceptively marketing Bluebonnet Chelated Magnesium, a magnesium supplement containing a blend of glycinate and oxide. Specifically, the complaint alleges that the company misleadingly labels the product because it does not list oxide as an ingredient on the product label, in violation of FDA regulations. (Hoffman et al v. Bluebonnet Nutrition Corp. and Albion Laboratories, Inc., Case No. 14-cv-00773, M. D. FL.).
For more information about other class-action lawsuits filed against Bluebonnet Nutrition and TINA.org’s coverage of the company, click here.
Class-Action Tracker

The Latest

Home Depot’s Rental Damage Protection Fee
TINA.org staffer gets surprise charge.

U-Haul Taking a Bite Out of the Big Apple
TINA.org files complaint with NYC over company’s “$19.95” truck rentals.

Costco Rotisserie Chicken: ‘No Preservatives’
Lawsuit cries fowl over preservative-free claims.
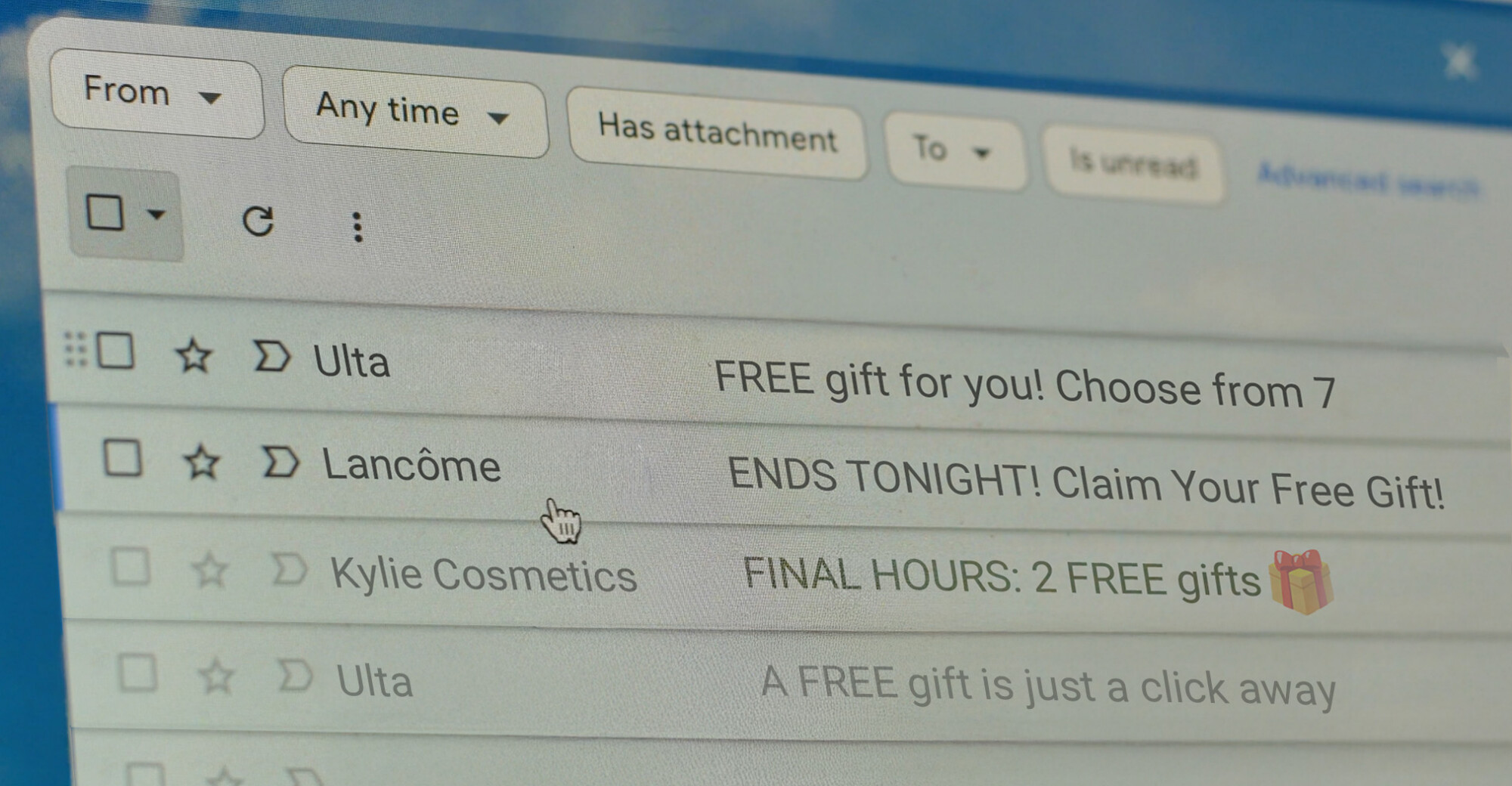
CATrends: Emails Offering Free Gifts
Lawsuits target misleading subject lines.

Keurig Reported to Regulators for Deceptively Marketing K-Cups as Recyclable
MADISON, CONN. Jan. 27, 2026 – Beverage giant Keurig Dr Pepper is deceptively marketing its single-serve K-Cup pods as “recyclable” in violation of state and federal laws, according to an…