FTC’s Negative Option Rule Needs Improvement
TINA.org applauds proposed amendments but pushes for more.
TINA.org strives to educate clinicians about patient testimonials.
| Laura Smith
When TINA.org encounters false or deceptive marketing campaigns, we typically use a two-pronged approach to address the problematic advertising: strive to educate consumers (through our investigative journalism and outreach) and get the deception to stop (by contacting the companies/individuals responsible and, when necessary, filing complaints with government regulators).
Generally, this approach is effective. In the overwhelming majority of cases in which TINA.org sends warning letters to companies and/or files complaints with government regulators regarding such companies, the deceptive marketing at issue is stopped. And in many cases, consumers receive some financial restitution (as a direct result of TINA.org actions, state and federal agencies have fined companies close to $250 million and returned millions in ill-gotten gains to consumers).
That said, every marketing campaign is different and sometimes TINA.org needs to go beyond its education/advocacy one-two punch.
One example of this is TINA.org’s 2018 investigation of the marketing used by the 50 cancer treatment centers in the U.S. that spent the most money on advertising in the previous calendar year.[1] TINA.org found that 90 percent of the cancer centers deceptively used patient testimonials to promote anecdotal, atypical patient results without clearly and conspicuously disclosing what the generally expected results for a patient in a similar situation would be (as is required by FTC law). Specifically, TINA.org’s investigation found hundreds of testimonials featuring patients with cancer types that have a less than 50 percent five-year survival rate, being used in direct-to-consumer marketing materials to advance the narrative, either explicitly or implicitly, that treatment at a specific cancer center will provide patients with a therapeutic advantage, allowing them to beat the odds and live beyond five years. Moreover, within this sampling of deceptive testimonials many also promoted clinical trials (i.e., research endeavors with no guarantee of therapeutic benefit), as well as novel treatments, such as immunotherapy and/or experimental procedures, without clearly and conspicuously disclosing their limitations, risks and relative rarity.
As a result of these findings, TINA.org took a multifaceted approach. First, we sent letters to each of the cancer centers at issue, notifying them of TINA.org’s findings. Second, we filed a complaint with the FTC against one of the centers – for-profit Cancer Treatment Centers of American (CTCA) – which was previously investigated by the Commission in 1996 and subject to a consent order at that time that prohibited it from, among other things, using patient testimonials that misrepresent the typical experience of its patients. Third, TINA.org endeavored to educate this particularly susceptible class of consumers by publishing articles on our website and information on our social media platforms, as well as issuing a press release.
But we haven’t stopped there. Many patient testimonials used in the deceptive marketing materials at issue featured oncologists and other clinicians who participated in the patients’ care. As such, TINA.org is making efforts to educate clinicians because those who are aware of – and can identify – deceptive marketing issues can take steps to help protect patients from making critical healthcare decisions based on incomplete and misleading marketing information. Moreover, clinicians can choose not to participate in the creation or spread of deceptive marketing materials, as well as educate patients about which sources to rely on when seeking educational information with regard to treatment options, survival rates, and general expectations.
Example of a patient testimonial deceptively used to promote a cancer center also featuring the treating oncologist:


According to the National Cancer Institute, Stage 4 lung cancer patients have a 5.8 percent five-year survival rate, as of May 2020. (At the time of publication, the five-year survival rate was 4.7 percent.)
Dr. Morgensztern is featured in the video discussing Kelly’s high quality of care and the clinical trial she participated in, and promoting Siteman Cancer Center:
One of the first things that we do when we see patients is that we try to detect whether they have a targetable mutation or not, and we’ve been very lucky with Kelly because we found this mutation and that led us to ask her to participate in this clinical trial…It is very important in my opinion to have the Siteman Cancer Center…
Kelly R. passed away in July 2018, three years after she was diagnosed. Siteman Cancer Center has since removed her testimonial from its website.
Example of a patient testimonial deceptively used to promote a cancer center also featuring the treating neurosurgeon:

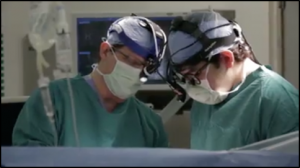
Denham Turner, Brain Cancer Survivor and Houston Methodist Neurosurgeon David S. Baskin, M.D.
According to the National Cancer Institute, brain cancer patients have a 32.6 percent five-year survival rate, as of May 2020. (At the time of publication, the five-year survival rate was 33.2 percent.)
Dr. Baskin is featured in the video discussing Denham’s successful brain cancer treatment at Houston Methodist:
So Denham is a triumph of surgical technology using that very precise neural navigation virtual reality system in the OR, we were able to go deep into his brain in areas that are very, very important for function, and very selectively get at the tumor and remove it without damaging the surrounding normal brain. A triumph of surgical technique and surgical technology combined with all of the treatment he had afterward to be sure the tumor hasn’t come back. … Every time he has a scan… it’s been okay and okay and okay and okay for years and years and years, so we’ve taken a very stressful, anxious situation and converted into a great triumph.
Dr. Morgensztern and Dr. Baskin, trained in medical oncology and neurosurgery, respectively, and presumably not advertising law or marketing, are not alone in being used by their employers to deceptively market cancer centers to a susceptible population of cancer patients. Thus, in May 2020, TINA.org’s executive director Bonnie Patten and I, together with a group of medical professionals that includes Fay J. Hlubocky, PhD, MA, Daniel F. McFarland, DO, Patricia A. Spears, BS, Jeffrey Peppercorn, MD, MPH, and Randall Holcombe, MD, MBA, published a paper in the 2020 American Society of Clinical Oncology (ASCO) Book entitled Direct-to-Consumer Advertising for Cancer Centers and Institutes: Ethical Dilemmas and Practical Implications. In this publication, we strive to educate the medical oncology community about the popular use of – and inherent risk in – patient testimonials, and join our co-authors in making recommendations for oncologists and cancer centers to ensure that marketing to patients is not deceptive or misleading.
Recommendations include, among others, refraining from including specific outcomes in patient testimonials that are atypical, and providing data and statistics about new technologies and approaches. We also recommend the avoidance of deceptive information that induces patients to travel for a clinical trial only to have the patient realize there is no viable option available for them. As noted above, many of the cancer centers TINA.org investigated used deceptive testimonials that promote clinical trials without clearly and conspicuously disclosing their limitations, risks and relative rarity. Meanwhile, many patients are traveling great distances to receive cancer care. (According to CTCA, nearly 70 percent of its patients travel to one of its hospitals from another state or country, traveling an average of 300 to 500 miles by plane, train or automobile.) Thus, clinicians must be vigilant not to induce sick patients to leave their homes and families, whether inadvertently or not, for clinical trials they may not even qualify for without providing all of the relevant information.
TINA.org will also be presenting on this topic at ASCO’s Annual Meeting in August 2020. Our goal in addressing clinicians – who are, generally speaking, neither the aggrieved consumer nor the responsible marketer – is to educate and warn the unsuspecting middleman, the person used by the cancer center to add weight and credibility to the patient’s account of their incredible recovery. This additional component to our typical one-two punch approach is critical. Not only can this deceptive marketing tactic negatively impact the physician-patient relationship and potentially put clinicians at risk and expose them to legal liability, but the marketing of false hope via patient testimonials can have serious and devastating results for patients and their families.
But there is reason to be hopeful: I have hope that through these multifaceted efforts, cancer centers will eventually do the right thing, stop using patient testimonials that deceive consumers, and provide potential clients with the data-driven information they need to make appropriate healthcare decisions.
_____________________________________________________________
[1] Other examples in which TINA.org went beyond its typical one-two punch include:
Both of these investigations ultimately led the FTC to file lawsuits against the companies in federal court, the former resulting in a $3.68 million settlement agreement with the company, and the latter resulting in a successful appeal to the Second Circuit Court of Appeals (with TINA.org’s support as amicus curiae).
TINA.org applauds proposed amendments but pushes for more.
New York neurosurgery center features deceptive testimonial on billboards and website
How deceptive MLMers try to evade regulators in the COVID era.