Most Deceptive Ads of 2024
Here were some of the worst ads TINA.org investigated this year.
Proceed with caution when it comes to menopause marketing.
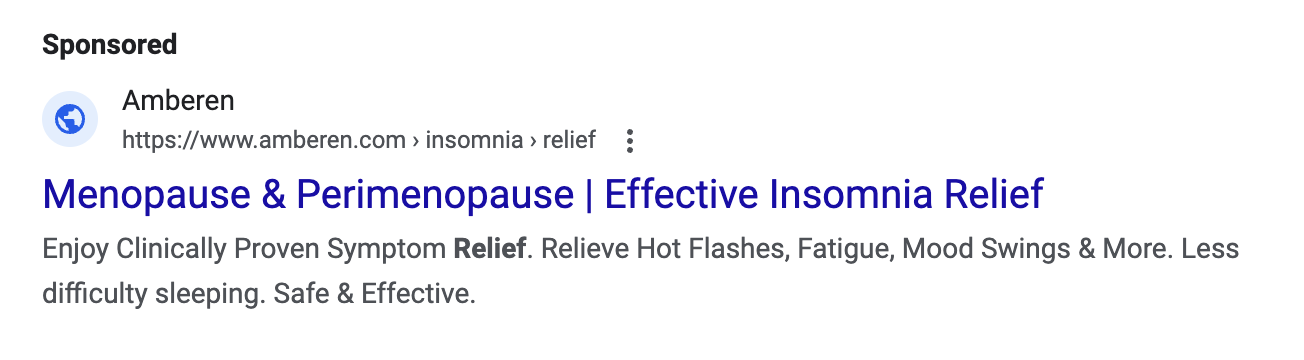
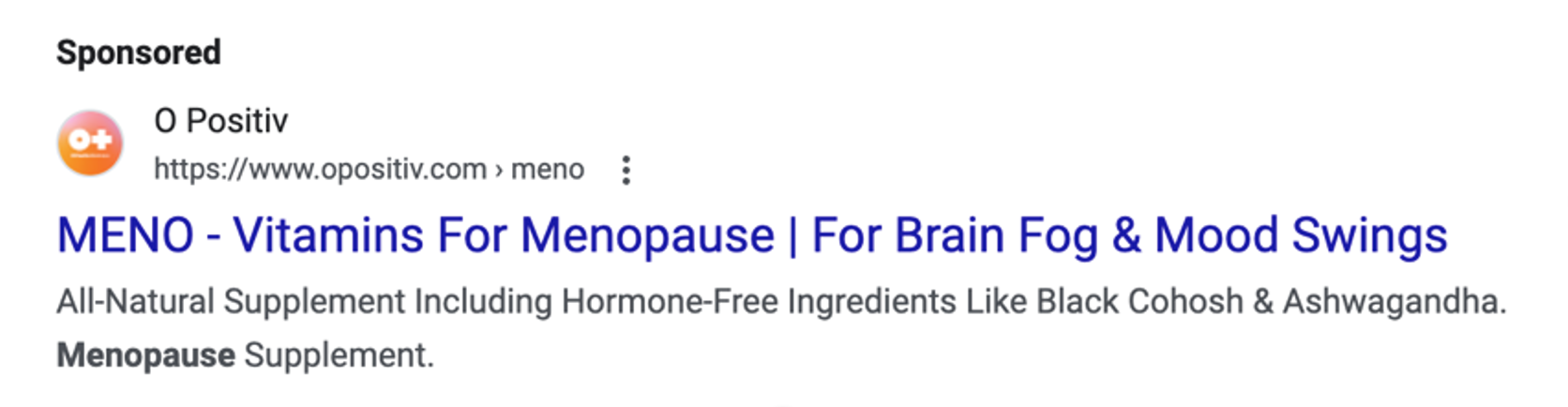
Millions of U.S. women* approaching or experiencing menopause (a transition often marked by a variety of disruptive symptoms ranging from hot flashes and nights sweats to anxiety, depression and low libido) are now the targets of ads for everything from FDA-approved drugs, to supplements, creams, essential oils, teas and even granola bars. The market for products that are advertised to ease menopause symptoms has grown to be a multibillion-dollar industry, with supplements claiming the lion’s share.
Unfortunately, according to medical experts, including at the National Institutes of Health and the Menopause Society, supplements have not been found to be effective at treating the symptoms of menopause. What’s more, many menopause supplement companies are making menopause relief claims in marketing materials without proper scientific support and without proper approval, in violation of FTC and FDA law.
So before you decide to purchase a menopause supplement, it’s worth carefully considering whether the marketing claims are simply too good to be true. Here are some tips to consider when evaluating menopause supplement marketing:
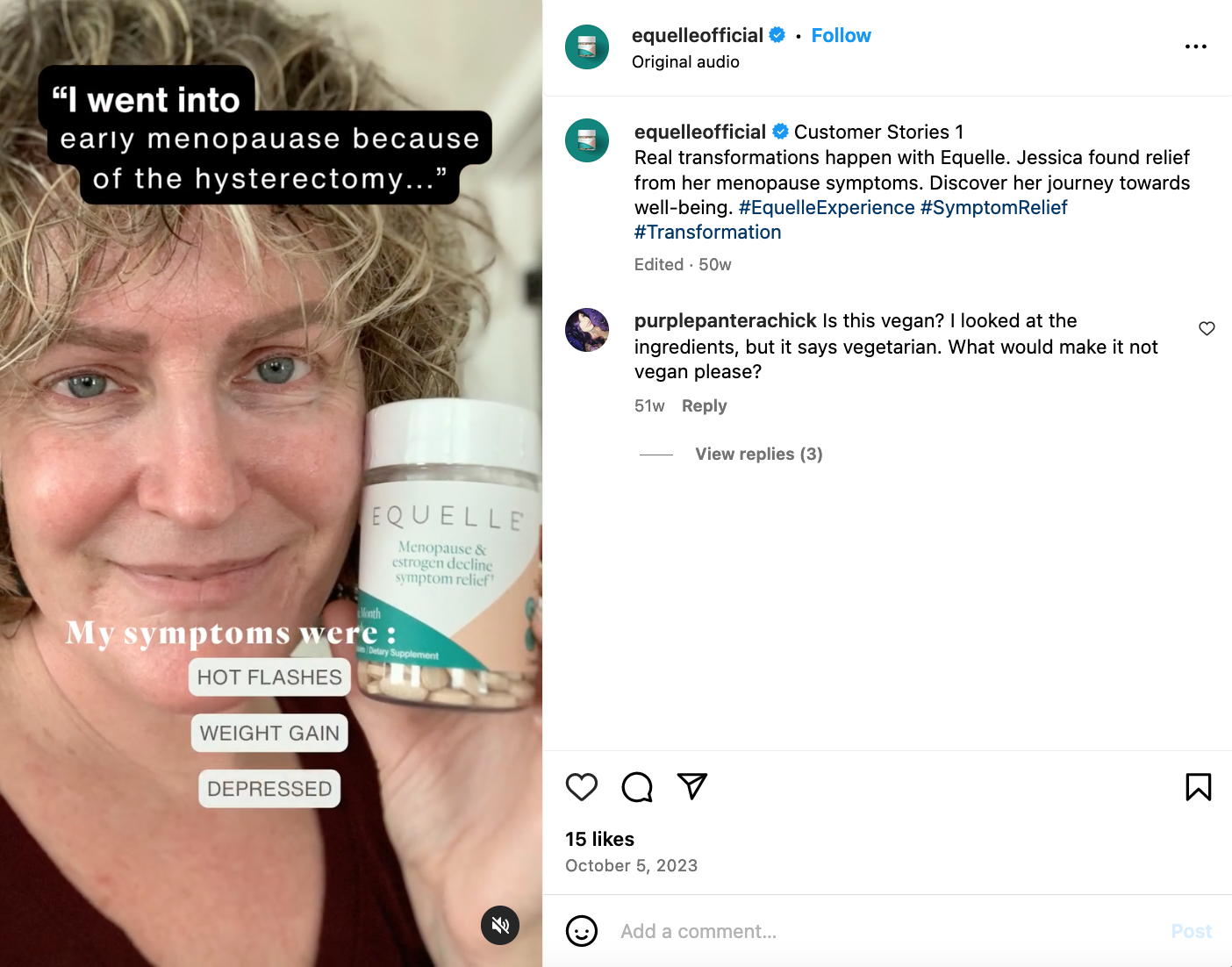
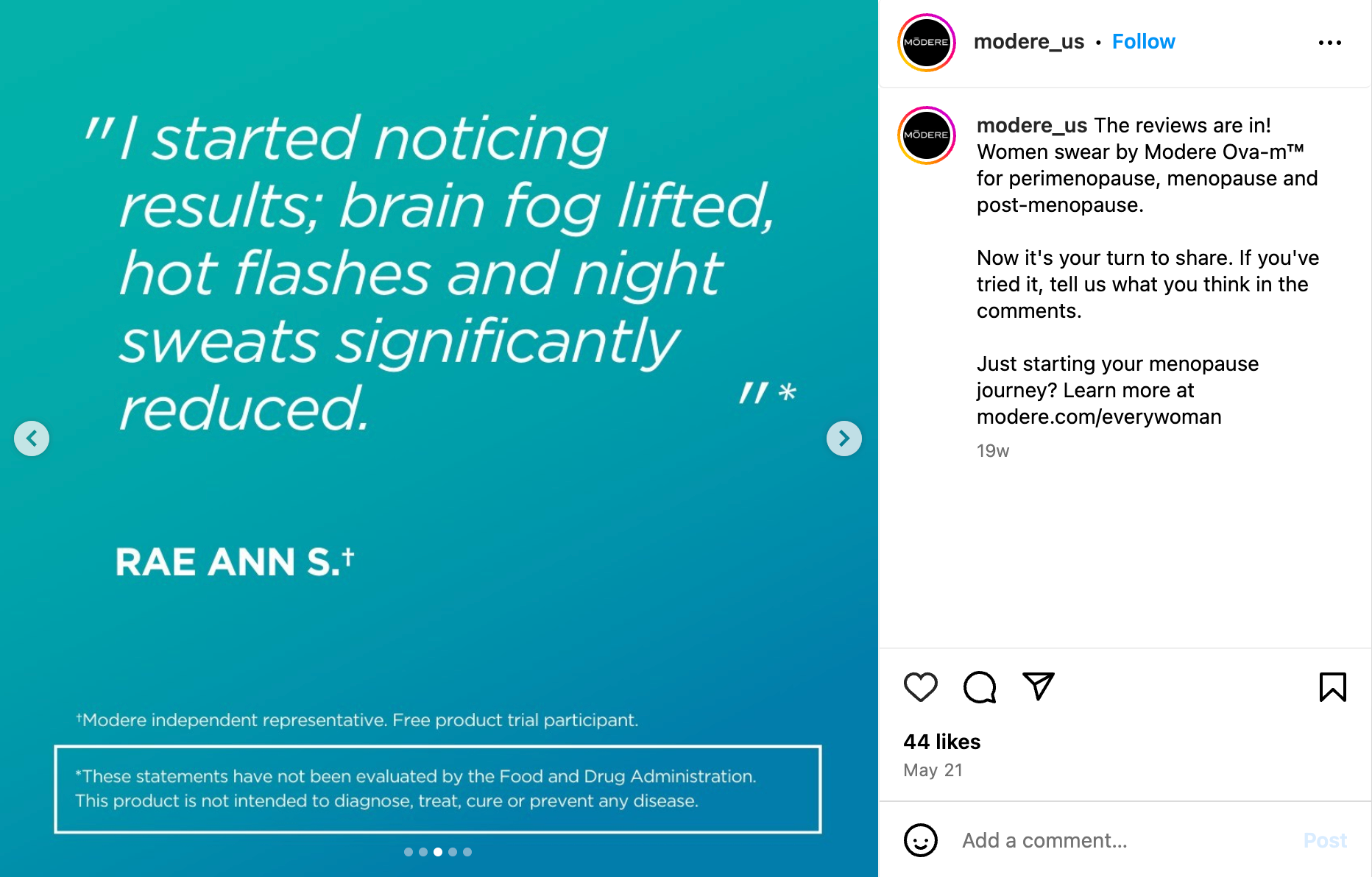
Beware of glowing customer testimonials.
Consumer testimonials about how a product has helped with menopausal symptoms can be compelling but there are a host of reasons why you should remain skeptical about testimonials. For example, there is a plethora of scientific literature showing that women of different races experience menopause differently. Then there’s the placebo effect, which is a significant issue when it comes to menopause aids, especially as it relates to hot flashes, depression, anxiety and pain.
Know that not all “clinical studies” validate ad claims.
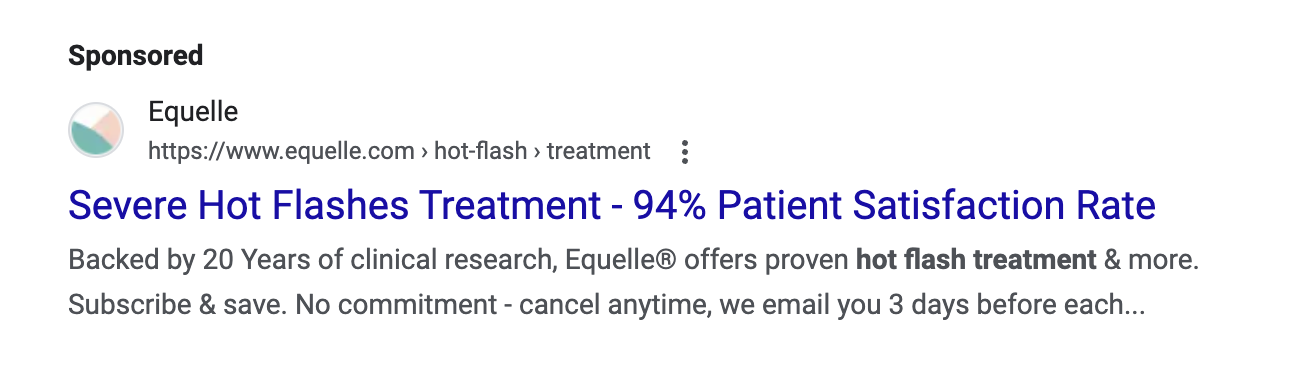
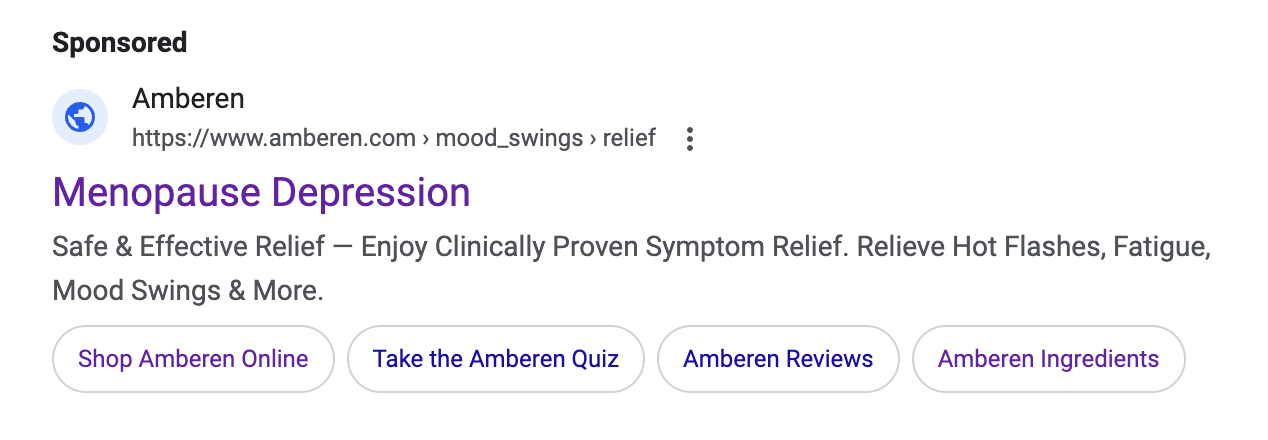
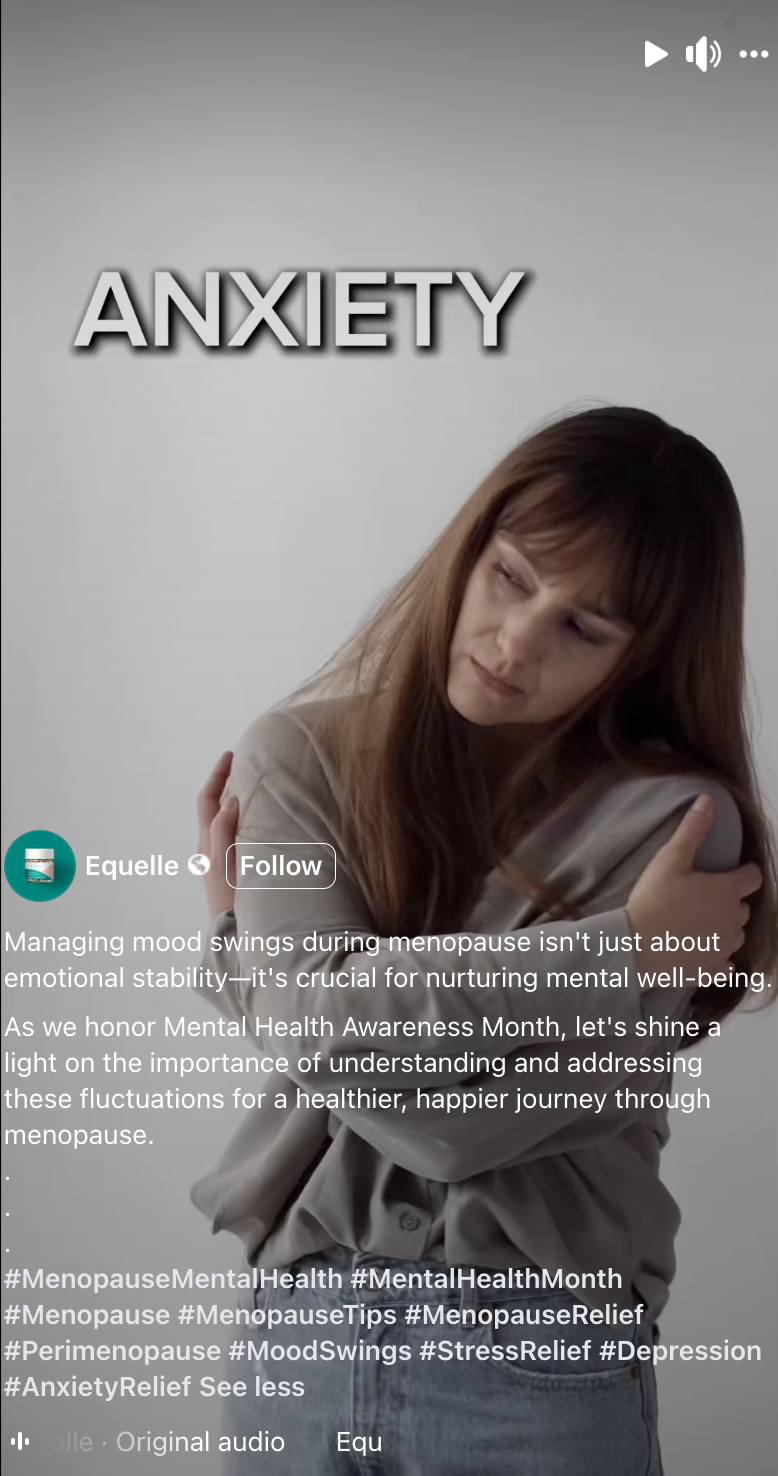
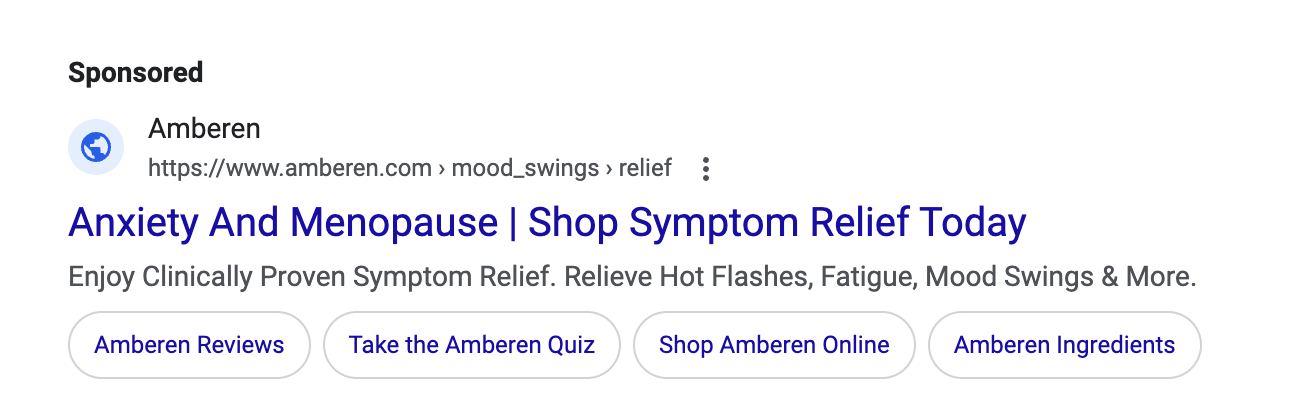
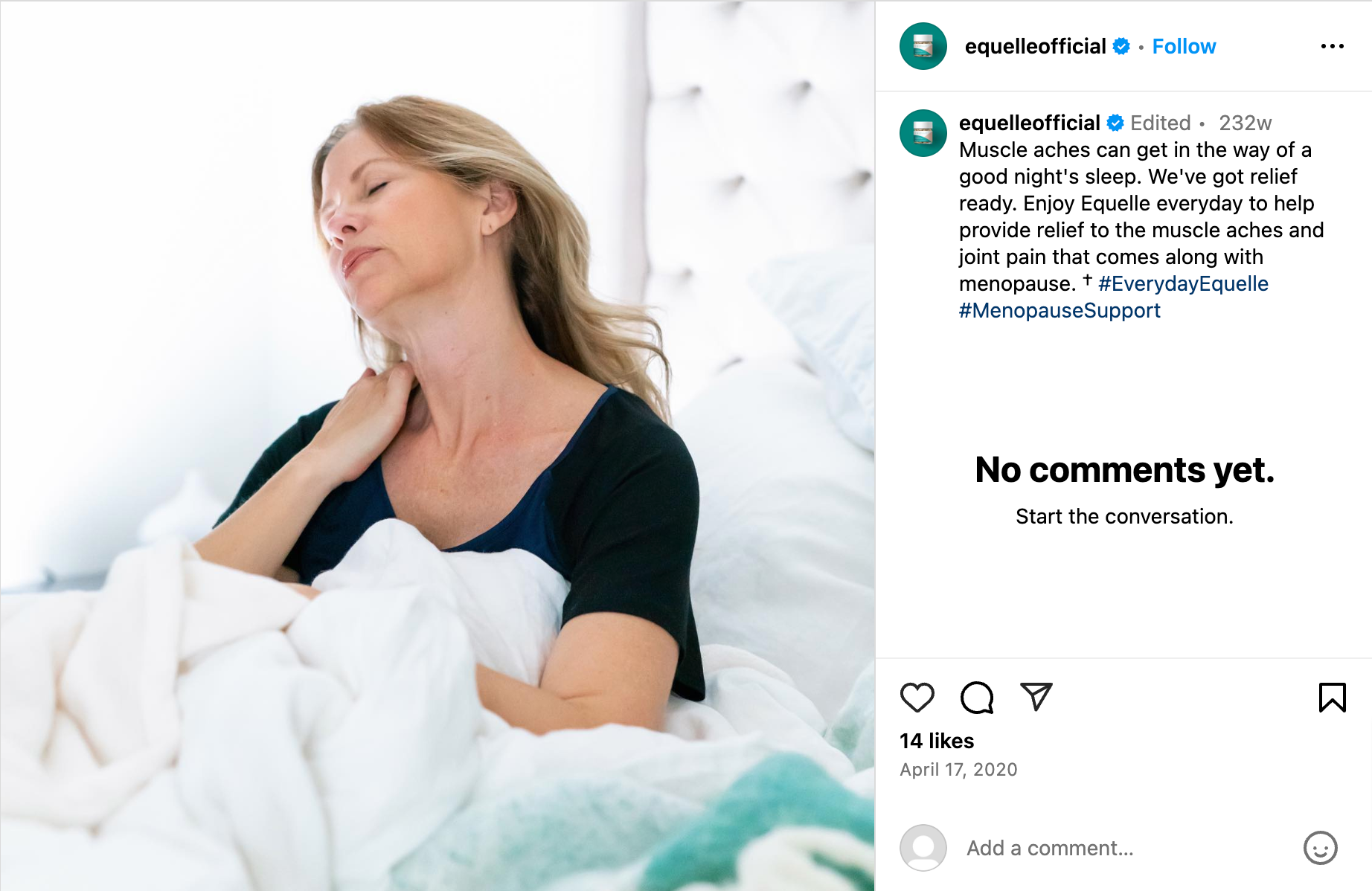
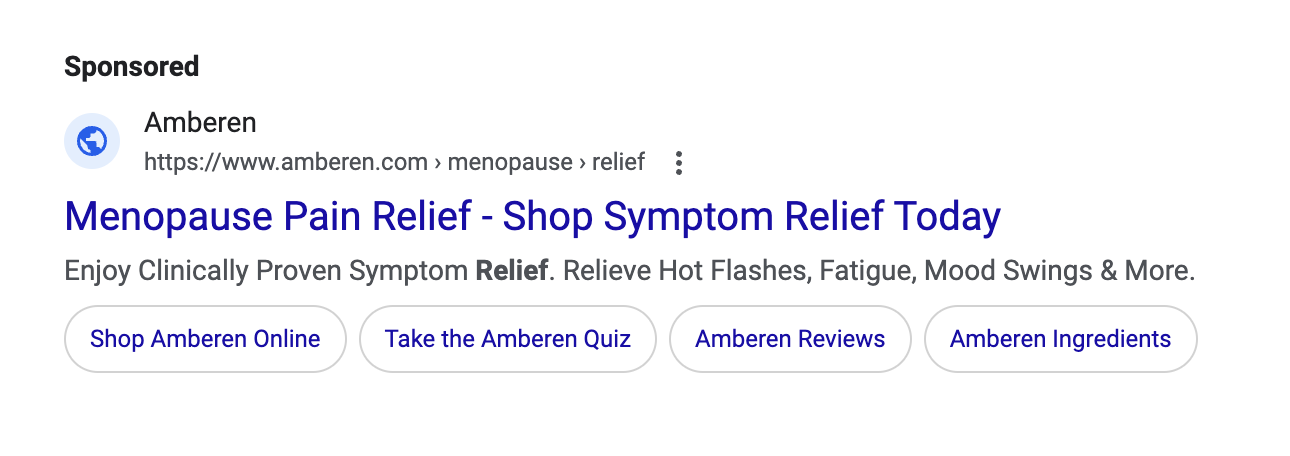
Just because a marketer says it has clinical or scientific evidence does not mean the marketing claims being made are properly backed-up. TINA.org has found that many “clinical studies” have major flaws that prevent them from properly supporting the marketing claims being made. In fact, TINA.org’s investigations into menopause supplements Equelle and Amberen found just that – marketing claims that simply were not properly supported by their studies.
Remember the FDA does not approve supplements.
No menopause supplement has FDA approval. Yet TINA.org has seen menopause supplement marketers use marketing claims that the federal agency has signaled should only be used to market FDA-approved drugs. Those claims – along with examples of those claims being used in marketing for supplements – include:
So if you see ads claiming that a supplement can do any of the above, proceed with extreme caution.
Look out for menowashing.
When it comes to menopause supplements, many brands like to tout that they are empowering women by offering them a real solution and allowing them to take control of their lives. But far from empowering or saving women, menowashing – the practice of marketing products as able to bring menopause relief without adequate support – exploits susceptible women struggling with menopause and looking for help.
Consult with a healthcare provider.
Menopause is a natural process but that doesn’t mean it doesn’t come with some problematic, and sometimes really debilitating, conditions and symptoms, like severe hot flashes that are so intense that they interfere with everyday activities. Instead of relying on marketing messages, talk to a healthcare professional about your menopausal symptoms to ensure you do what’s best for you.
What TINA.org is doing
TINA.org has taken steps to eradicate deception in the menopause supplement industry by filing complaints with the FTC and FDA against Equelle and Amberen. TINA.org has also sent letters to 100 other menopause supplement companies notifying them of this deceptive marketing trend in their industry and urging them to review their menopause marketing. It’s time that the menopause supplement industry takes a long, hard look at its marketing and ensures that it is honest, truthful and legal.
TINA.org will be watching and if you’ve seen or heard a questionable menopause supplement promotion, we want to hear from you too.
—
*While TINA.org understands and appreciates that individuals who do not identify as women may also experience menopause, the term women is used to reflect the marketing at issue.
Here were some of the worst ads TINA.org investigated this year.
An investigation into the menopause supplement industry by consumer advocacy organization truthinadvertising.org (TINA.org) has revealed a hotbed of deceptive advertising. The ad watchdog has amassed nearly 2,000 examples of problematic health…
How the supplement industry is taking advantage of women and what TINA.org is doing to fight it.