Alkaline Water Plus
Are there really any benefits to drinking alkaline water?
FDA warns company about serious violations including unapproved drug claims.
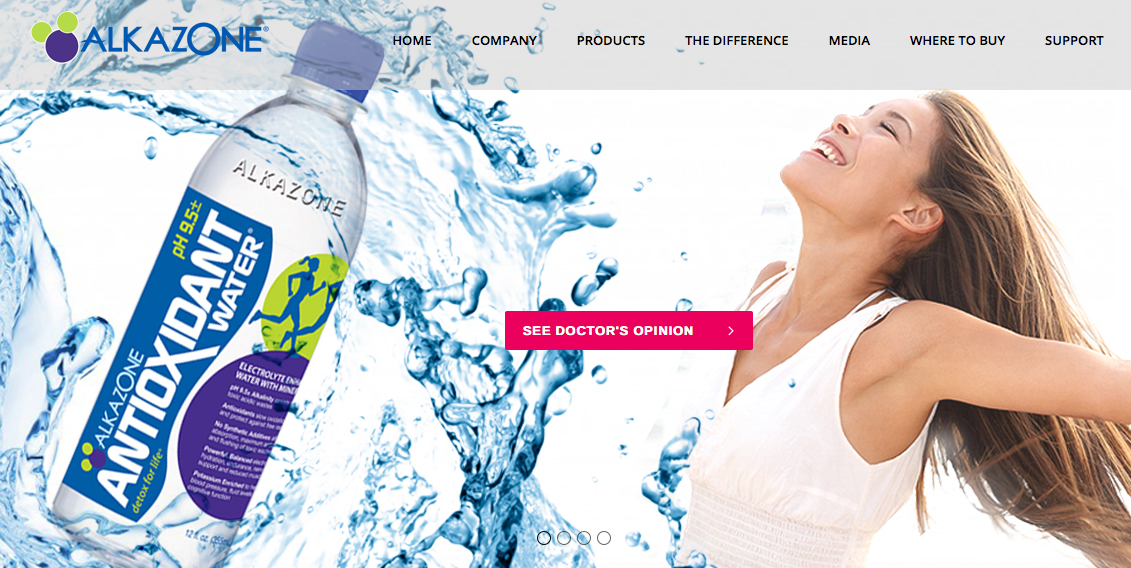
Interested in a “doctor’s opinion” on Alkazone Antioxidant Water? You’re not getting it — at least not from the product’s website anymore.
That’s because the link-in-pink on alkazone.com (see screenshot above, taken Feb. 27) now takes visitors to a page that no longer exists. Why does it do this? Most likely because the FDA wanted the video gone. In a recent letter to Better Health Lab, the manufacturer and marketer of Alkazone, the agency warned that the doctor’s video (in addition to other areas of the website) contained unapproved drug claims regarding Alkazone products.
So they got the message, right? Well, maybe not. As of this writing, many of the problem claims cited in the letter still appear on the site, including claims that Alkazone Premium Energy Booster “normalizes blood pressure” and “reduces the risk of heart disease.”
Check with your doctor before taking any supplement. And for more of our coverage on products cited by the FDA for making unapproved drug claims, click here.
Our Ad Alerts are not just about false and deceptive marketing issues, but may also be about ads that, although not necessarily deceptive, should be viewed with caution. Ad Alerts can also be about single issues and may not include a comprehensive list of all marketing issues relating to the brand discussed.
Are there really any benefits to drinking alkaline water?
TINA.org breaks down the legal issues of marketing a “hangover supplement.”
How Amazon steers consumers toward unproven and potentially dangerous products containing a fake vitamin called B17.