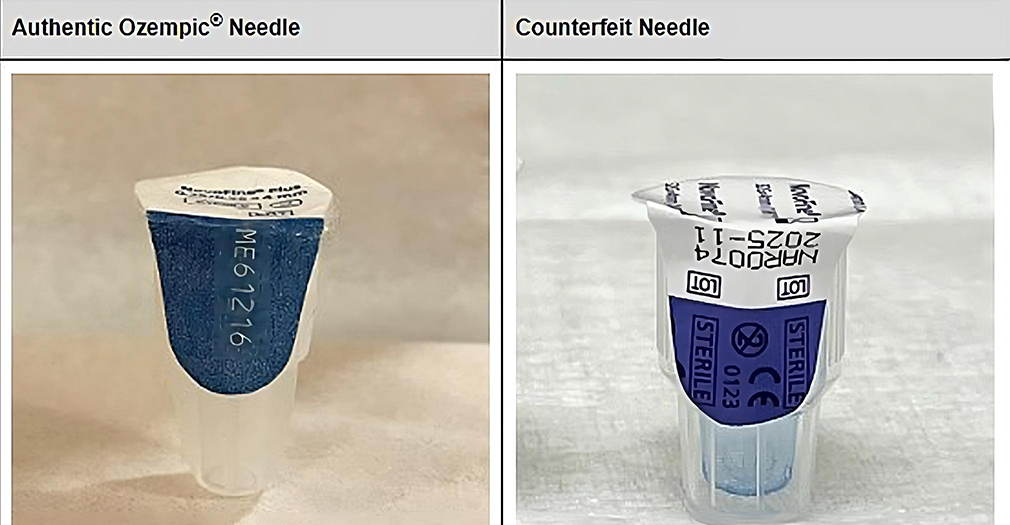
Counterfeit Ozempic
FDA says it has seized “thousands of units” of fake shots.
Our Ad Alerts are not just about false and deceptive marketing issues, but may also be about ads that, although not necessarily deceptive, should be viewed with caution. Ad Alerts can also be about single issues and may not include a comprehensive list of all marketing issues relating to the brand discussed.
FDA says it has seized “thousands of units” of fake shots.
A cascade of deceptive marketing issues.
Unapproved drug treatment claims remain in wake of FDA warning letter.
Following lawsuit, chain adds more detailed disclosures about caffeine content.
This ad hits different when you read the fine print.
FDA targets companies selling eye drops illegally marketed to treat conditions like pink eye.
Company misleadingly markets lozenges as an effective nasal decongestant, according to lawsuit.
Supplement company doesn’t have the proper scientific evidence to back up its health claims.
Counting up the issues with this purported autism supplement.
Deceptive mailer targets Medicare recipients.








