Bespoke Apothecary
Products marketed to treat COVID are pulled in wake of FDA/FTC warning letter.
Stephens et al. v. Laboratory Corporation of America Holdings
23-cv-4266, E.D.N.Y.
(June 2023)
MaterniT Genome noninvasive prenatal screening test
Falsely advertising that tests are able to detect chromosomal abnormalities with “a high degree of accuracy” when the results are not reliable for numerous conditions
Pending
Products marketed to treat COVID are pulled in wake of FDA/FTC warning letter.
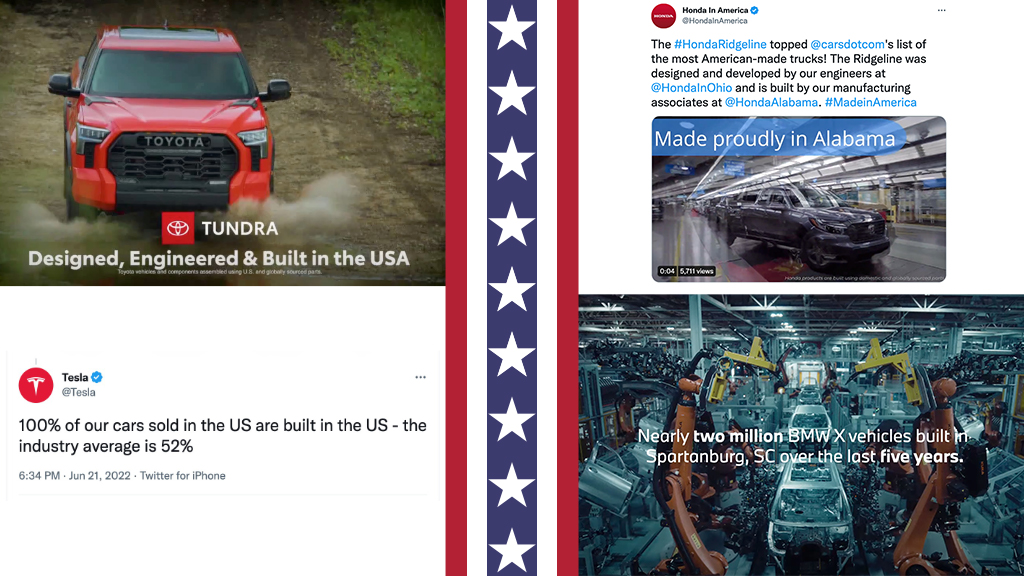
MADISON, CONN. November 7, 2022 – No Super Bowl is complete without at least one car commercial teeming with red, white and blue flags and blatant nods to Americana with…
Jury puts a dollar value on a competitor’s false U.S.-origin claims.
Not according to the cooking instructions on the side of the box.
TINA.org puts the auto industry on notice.